 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 22(4); 2022 > Article |
|
Abstract
Autoimmune gastritis (AIG) is a type of atrophic gastritis characterized by destruction of parietal cells in the gastric fundus and body. These changes may be attributable to immune-mediated chronic inflammatory responses. AIG is characterized by extensive atrophy of the gastric body; therefore, endoscopic findings offer useful diagnostic clues. AIG is diagnosed based on serological and histopathological evaluation of endoscopic biopsy specimens; however, this condition may be accompanied by autoimmune diseases including autoimmune thyroid disease (ATD), and the opposite can be suspected. Diagnostic delays and misdiagnosis are common in patients with AIG owing to the nonspecific clinical presentation and accompanying autoimmune diseases. Additionally, confirmation of AIG based on serological atrophy or endoscopic findings is challenging in cases of active Helicobacter pylori (H. pylori) infection. We report a case of Graves’ disease (an ATD) in a patient diagnosed with AIG and concomitant H. pylori-induced gastritis based on the rapid urease and serological test results and endoscopic biopsy findings.
The intestine, stomach, and thyroid gland share a common embryonic origin; therefore, a presentation of autoimmune thyroid disease (ATD) such as Hashimoto’s thyroiditis and Graves’ disease accompanied by autoimmune gastritis (AIG) or celiac disease, was previously referred to as “thyrogastric syndrome.” [1,2] AIG is an immune-mediated disease in which auto-activated T cells react with antibodies that recognize the H+/K+ ATPase in parietal cells (which secrete gastric acid) and trigger a persistent inflammatory response, which leads to gastric corpus atrophy [3]. Gradual parietal cell destruction subsequently results in a significant decline in gastric acid and intrinsic factor secretion, with consequent iron, vitamin B12, and micronutrient deficiencies that clinically manifest as anemia, sensory and peripheral neuropathy, infertility, and repeated miscarriages [4]. Extensive histopathologic evolution of gastric corpus atrophy is accompanied by injury to chief cells and increased gastrin cell expression, which result in a significant decline in serum pepsinogen (PG) I levels and the PG I/II ratio and elevated serum gastrin levels, which characterize serological atrophic gastritis [3-5]. However, AIG is typically asymptomatic clinically; therefore, most patients are misdiagnosed with functional dyspepsia, gastroesophageal reflux disease, or anemia, which leads to diagnostic delays [6]. Endoscopic biopsy to confirm gastric corpus inflammation serves as the diagnostic gold standard for AIG; however, serum testing and a confirmatory anti-parietal cell antibody (APCA) test may be performed in patients with a high index of clinical suspicion for an autoimmune disease (AD) accompanying AIG [7]. In this case report, we describe a patient with Graves’ disease diagnosed with AIG and concomitant Helicobacter pylori (H. pylori)-induced gastritis based on positive APCA and rapid urease test results and serological atrophy with histopathological evaluation of endoscopic biopsy specimens for diagnostic confirmation.
A 44-year-old woman diagnosed with Graves’ disease approximately 8 years before study enrolment was administered radioactive iodine, which led to hypothyroidism for which she was treated with synthroid (0.01 mg). Prior to initiation of radioactive iodine therapy, the patient received a blood transfusion for management of severe iron-deficiency anemia during pregnancy and was intermittently administered iron supplements. She did not have gastrointestinal symptoms and denied a history of gastric acid secretion inhibitor use or treatment for H. pylori.
Blood tests performed for evaluation of anemia and thyroid hormone levels showed the following results: serum hemoglobin 7.5 g/dL (reference range, 12.0~16.0), hematocrit 25.1% (37.0~40.0), mean corpuscular volume (MCV) 73.2 fl (80.0~100.0), mean corpuscular hemoglobin (MCH) 21.6 pg (27.0~34.0), MCH concentration 29.5 g/dL (32.0~36.0), serum iron 13.0 µg/dL (33.0~193.0), ferritin 8.10 ng/mL (13.0~150.0), transferrin and ironbinding capacity 371.0 µg/dL (228.0~428.0), and vitamin B12 of 403.0 pg/mL (180.0~914.0). Therefore, the patient was diagnosed with iron-deficiency anemia. Her thyroid panel test results showed a free thyroxine level of 1.32 ng/dL (reference range, 0.85~1.50), triiodothyronine 1.62 ng/mL (0.58~1.62), thyroid stimulating hormone (TSH) <0.05 µIU/mL (0.25~5.00), TSH receptor antibody 2.26 IU/L (0.0~1.22), anti-thyroid-peroxidase antibody 10.1 IU/mL (0.0~34.0), and thyroglobulin antibody 13.9 IU/mL (0.0~115.0), all of which indicated well-maintained thyroid gland function following radioactive iodine therapy. We observed no specific gastrointestinal symptoms and performed serological testing to confirm accompanying AIG. Results showed serum PG I and II levels of 28.7 ng/mL and 26.3 ng/mL, respectively with a PG I/II ratio of 1.1, which indicated an extremely low PG I level and PG I/II ratio with the serum gastrin level elevated to 792.0 pg/mL (13.0~115.0). Anti-H. pylori antibodies measured using the H. pylori IgG Immulite® 2000 analyzer (Diagnostic Products, Los Angeles, CA, USA) showed a positive result of 4.27 U/mL (negative <0.90 U/mL, equivocal 0.90~1.09 U/mL, positive ≥1.10 U/mL). An APCA test using an immunofluorescence assay (Kallestad Mouse Stomach/Kidney; Bio RAD, Hercules, CA, USA) revealed positive results, and we suspected autoimmune thyroiditis accompanied by AIG.
Endoscopic evaluation to confirm AIG and concomitant H. pylori infection, if any, revealed mild gastric antral atrophy (Fig. 1A) with prominent submucosal blood vessels in the gastric fundus and body, indicative of corpus-dominant atrophy with disappearance of gastric folds (Fig. 1B). H. pylori -induced spotty redness was observed in the greater curvature of the gastric body (Fig. 1C). Therefore, we performed the rapid urease test, which showed a positive result. Biopsies obtained from the gastric antrum and body showed some atrophic changes and inflammatory cell infiltration in the gastric antral mucosa (Fig. 1D), with severe mucosal atrophy at the greater and lesser curvatures of the gastric body and prominent inflammatory cell infiltration in the lamina propria (Fig. 1E). Immunohistochemical analysis using chromogranin revealed enterochromaffin-like cell proliferation in the gastric body (Fig. 1F). The patient was eventually diagnosed with AIG and prescribed iron supplementation and H. pylori eradication therapy and is followed up as an outpatient.
Patients with AIG show a 3~5 times higher risk of ADs, including ATD, type 1 diabetes mellitus, alopecia, celiac disease, vitiligo, connective tissue disease, and primary biliary cholangitis than that observed in the general population [3,8]. More than 50.0% of patients with AIG may develop AD, and ATD represents the most common comorbidity in approximately >36.0% of patients [9]. APCAs are most commonly accompanied by organ-specific autoantibodies [10]. Table 1 summarizes the ADs that may occur concomitant with AIG (from high to low prevalence rates) [8].
Graves’ disease, an ATD associated with hyperthyroidism is accompanied by anemia in >30.0% of patients. The chronic disease-like “Graves’ anemia” is the most common pattern of disease, in which patients maintain normal serum thyroid hormone levels post-treatment, and the condition improves in most cases [11]. Our patient did not undergo medical record review before initiation of radioactive iodine therapy for Graves’ disease; therefore, whether the patient had Graves’ anemia remains unclear. However, considering normal serum thyroid hormone levels post-treatment and significantly reduced MCV and ferritin levels, anemia at the time of her hospital visit was diagnosed as iron-deficiency anemia and not Graves’ anemia. Graves’ disease concomitant with iron-deficiency anemia results in increased APCA expression and elevated serum gastrin levels, which is characteristic of AIG [12]. Enterochromaffin-like cell proliferation may progress to neuroendocrine tumor formation in patients with ATD associated with AIG, elevated serum gastrin is diagnostically significant in patients who show these histopathological changes [9,11-13]. A serum PG test may be an important biomarker to confirm severe histological atrophy, regardless of H. pylori infection in patients with ATD [14]. PG I levels in our patient were higher than those usually observed in patients with AIG, which may be attributable to the fact that this patient presented with concomitant active H. pylori infection [15]. Although a serum PG test indicated serologic atrophy in our patient with Graves’ disease, AIG was suspected based on hypergastrinemia and was subsequently diagnosed following biopsy and immunohistochemical evaluation.
AIG, which is attributable to an immune-mediated chronic inflammatory response with concomitant H. pylori infection in most cases, is a known etiopathogenetic contributor to gastric cancer. Therefore, early diagnosis and timely management are important [16]. AIG is diagnosed based on positive APCAs or anti-intrinsic factor antibody test results, endoscopically documented gastric body atrophy, or detection of pernicious anemia, with accompanying elevation of serum gastrin levels [17]. However, early-stage AIG may present with normal serum PG test results and normal endoscopic findings [18]. A negative APCA test result cannot exclude diagnosis of AIG, and histopathological evaluation is essential for diagnostic confirmation [19].
A recent Korean study has reported AIG in five patients; however, diagnostic or therapeutic guidelines for ATD accompanied by AIG are unavailable [20]. AIG should be suspected and serological testing is warranted in patients with ATD accompanied by nutrient deficiencies that show varied clinical presentations, including anemia of unknown etiology. Anemia, serological atrophy, and elevated serum gastrin levels are significant markers for diagnosis of AIG; APCA assays and endoscopic biopsy evaluation should be performed in such cases for definitive diagnosis.
Fig. 1.
Endoscopic and histopathological findings. Mild atrophic changes are observed in the antral mucosa (A). Extensive atrophic changes with marked submucosal vasculature are observed in the mucosa of the fundus and gastric body (B). Focal disappearance of folds and spotty redness are observed in the greater curvature of the gastric body (C). Mild mucosal destruction accompanied by chronic inflammatory cell infiltration of the lamina propria is observed in the antrum (Hematoxylin and Eosin [H&E] stain, ×100) (D). Mucosal destruction with significant inflammatory cell infiltration of the lamina propria and focal intestinal metaplasia are observed in the gastric body (H&E stain, ×100) (E). Increased numbers of neuroendocrine cells with linear neuroendocrine cell hyperplasia are observed in the body (Chromogranin A stain, ×100) (F).
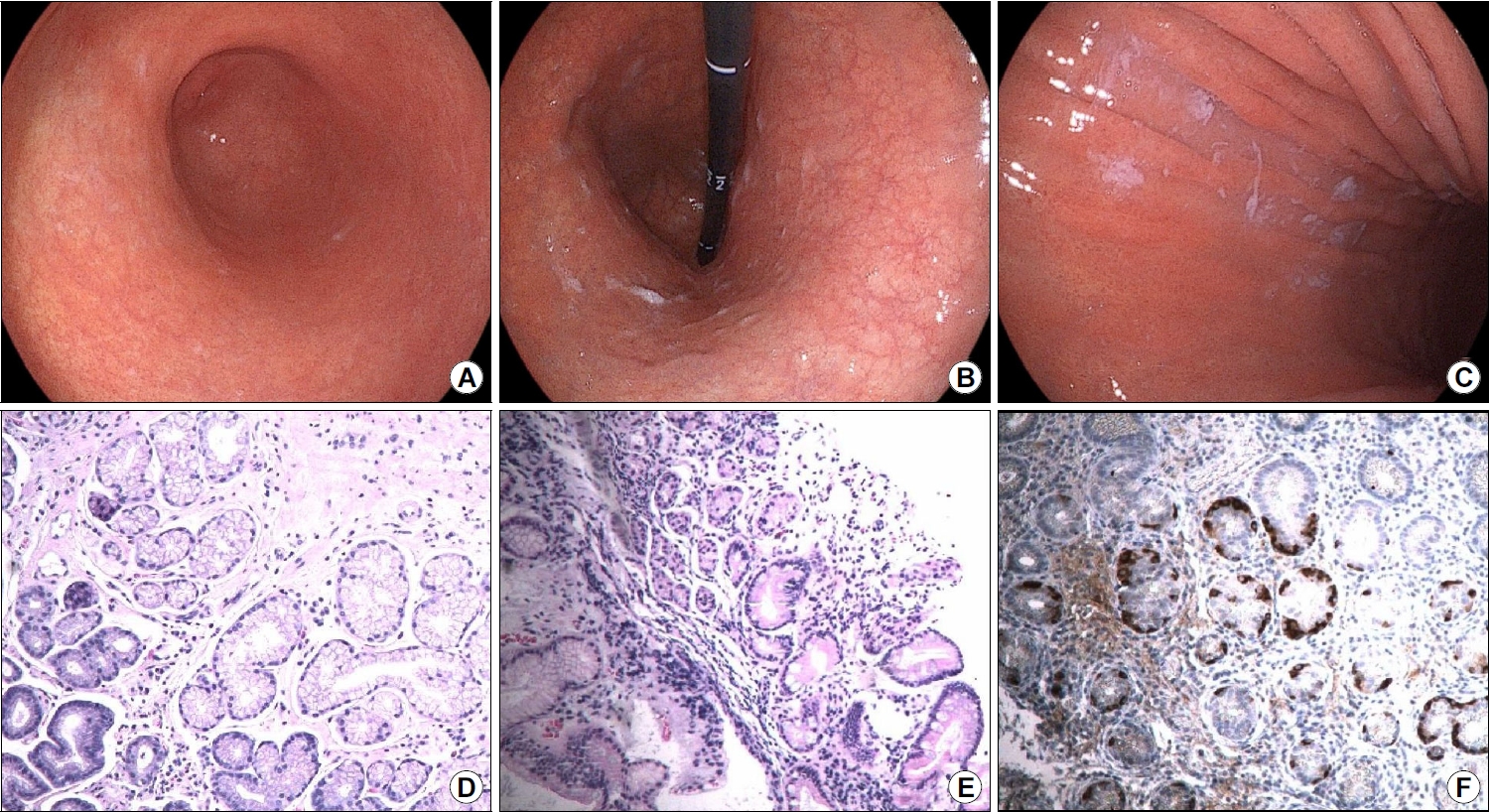
Table 1.
Autoimmune Diseases Accompanying Autoimmune Gastritis
| Association of comorbidities | Autoimmune diseases accompanied with autoimmune gastritis |
|---|---|
| Very higha | Autoimmune thyroiditis (Hashimoto’s thyroiditis) and Graves’ disease |
| Highb | Type 1 diabetes mellitus |
| Low | Alopeciac |
| Celiac diseasec | |
| Vitiligod | |
| Connective tissue diseasee | |
| Primary biliary cholangitise | |
| Very lowf | Myasthenia gravis |
| Primary sclerosing cholangitis | |
| Autoimmune hepatitis | |
| Addison’s disease | |
| Primary ovarian insufficiency | |
| Primary hypoparathyroidism | |
| Lambert-Eaton syndrome | |
| Oral erosive lichen planus |
REFERENCES
1. Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann 1980;9:154–162.


2. Lahner E, Conti L, Cicone F, et al. Thyro‐entero‐gastric autoimmunity: pathophysiology and implications for patient management. Best Pract Res Clin Endocrinol Metab 2020;34:101373.


4. Rustgi SD, Bijlani P, Shah SC. Autoimmune gastritis, with or without pernicious anemia: epidemiology, risk factors, and clinical management. Therap Adv Gastroenterol 2021;14:17562848211038771.



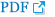
5. Zagari RM, Rabitti S, Greenwood DC, Eusebi LH, Vestito A, Bazzoli F. Systemic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther 2017;46:657–667.


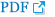
6. Lenti MV, Miceli E, Cococcia S, et al. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment Pharmacol Ther 2019;50:167–175.


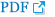
7. Rodriguez-Castro KI, Franceschi M, Miraglia C, et al. Autoimmune diseases in autoimmune atrophic gastritis. Acta Biomed 2018;89:100–103.


8. Massironi S, Zilli A, Elvevi A, Invernizzi P. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev 2019;18:215–222.


10. Pilli T, Dalmazio G, Porcelli B, et al. Screening of organ-specific autoantibodies in a large cohort of patients with autoimmune thyroid disease. Thyroid 2021;31:1416–1423.


11. Gianoukakis AG, Leigh MJ, Richards P, et al. Characterization of the anemia associated with Graves’ disease. Clin Endocrinol (Oxf) 2009;70:781–787.

12. Gianoukakis AG, Gupta S, Tran TN, Richards P, Yehuda M, Tomassetti SE. Graves’ disease patients with iron deficiency anemia: serologic evidence of co-existent autoimmune gastritis. Am J Blood Res 2021;11:238–247.


13. Nicolaou A, Thomas D, Alexandraki KI, Sougioultzis S, Tsolakis AV, Kaltsas G. Predictive value of gastrin levels for the diagnosis of gastric enterochromaffin-like cell hyperplasia in patients with Hashimoto’s thyroiditis. Neuroendocrinology 2014;99:118–122.


14. Venerito M, Radünz M, Reschke K, et al. Autoimmune gastritis in autoimmune thyroid disease. Aliment Pharmacol Ther 2015;41:686–693.


15. Kishikawa H, Nakamura K, Ojiro K, et al. Relevance of pepsinogen, gastrin, and endoscopic atrophy in the diagnosis of autoimmune gastritis. Sci Rep 2022;12:4202.



16. Rugge M, Bricca L, Guzzinati S, et al. Autoimmune gastritis: long-term natural history in naïve Helicobacter pylori-negative patients. Gut 2022 doi: 10.1136/gutjnl-2022-327827. [Epub ahead of print].

17. Terao S, Suzuki S, Yaita H. et al. Multicenter study of autoimmune gastritis in Japan: clinical and endoscopic characteristics. Dig Endosc 2020;32:364–372.

18. Kotera T, Yamanishi M, Kushima R, Haruma K. Early autoimmune gastritis presenting with a normal endoscopic appearance. Clin J Gastroenterol 2022;15:547–552.


-
METRICS

-
- 1 Crossref
- 2,227 View
- 60 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Vonoprazan-based Dual and Triple Therapy for Helicobacter pylori Eradication2023 September;23(3)
Antibiotic Resistance and Helicobacter pylori Eradication Therapy2023 September;23(3)






