|
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 24(1); 2024 > Article |
|
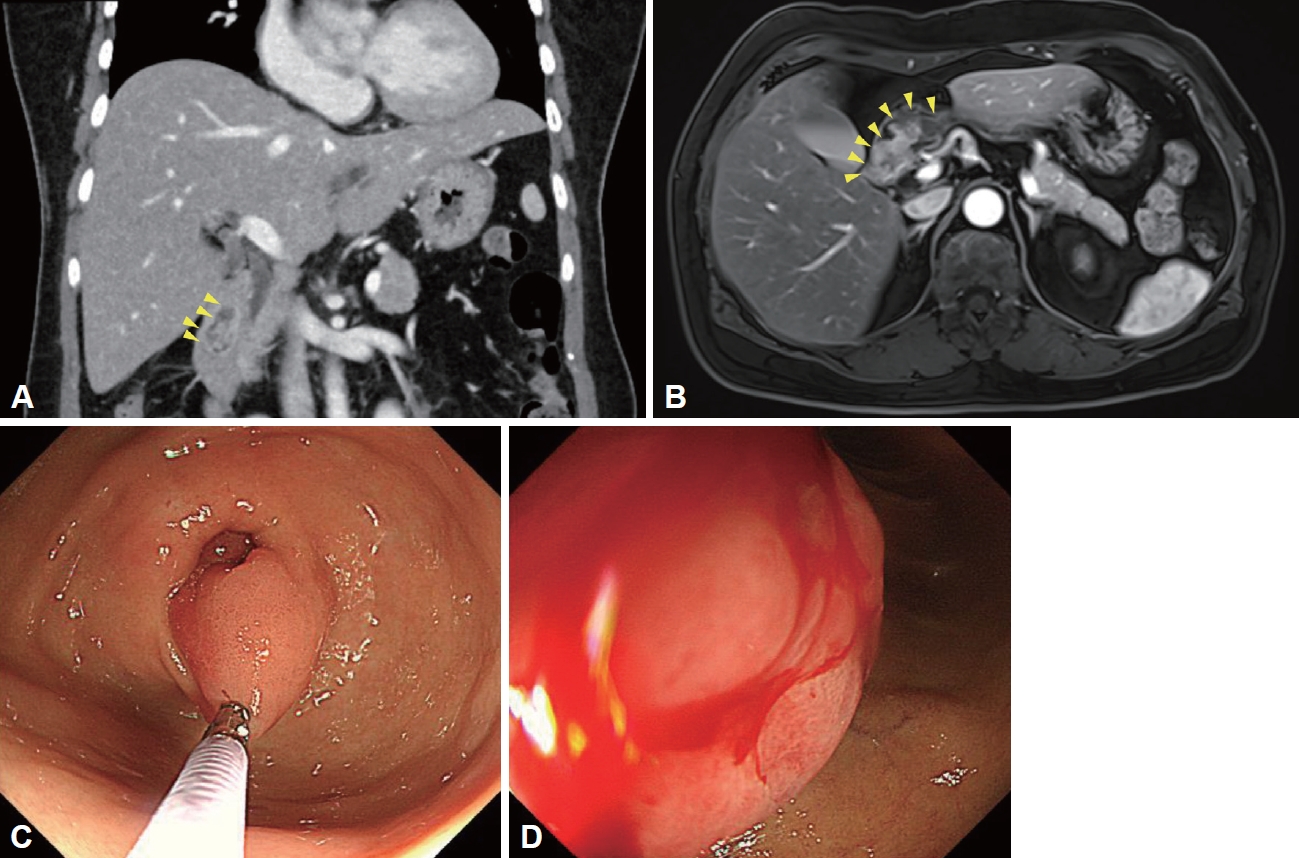
A 55-year-old woman visited the outpatient clinic to evaluate further a duodenal mass detected on the screening endoscopy. The patient complained an intermittent melena, and initial laboratory results showed microcytic hypochromic anemia (Hgb 10.0 g/dL [reference 12.0–16.0 g/dL], MCV 71.9 fL [reference 79.0–95.0 fL], MCHC 29.7 g/dL [reference 32.0–36.0 g/dL], and RDW 16.9% [reference 11.5%–14.5%]). On the abdomen computed tomography, about 2.7 cm-sized fat-containing enhancing mass was found in the duodenum (Fig. 1A). On the magnetic resonance imaging, the duodenal mass originated from the distal antrum (Fig. 1B). Endoscopy revealed a 2.5 cm-sized pedunculated hyperemic polyp that originated from the greater curvature of the pyloric ring (Fig. 1C). The head of the polyp was located at the bulb of the duodenum and was hyperemic with a spontaneously oozing hemorrhage (Fig. 1D). What is the most likely diagnosis?
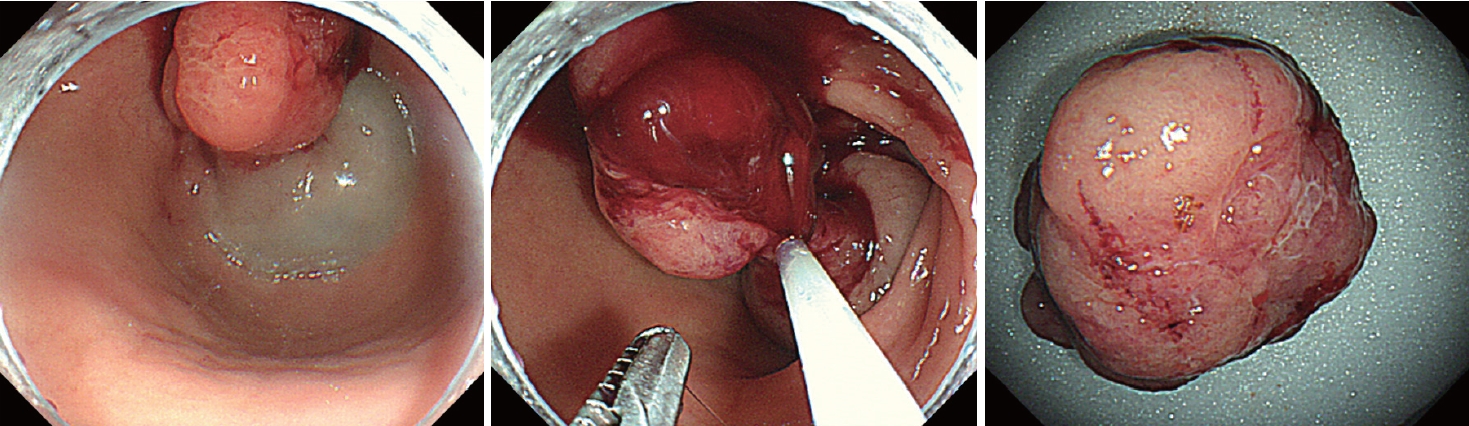
The endoscopic diagnosis of the polyp was a polyp causing anemia due to chronic gastrointestinal blood loss. An endoscopic biopsy from the polyp head revealed Helicobacter pylori-negative inflammatory polyp. An endoscopic mucosal resection (EMR) was performed to remove the polyp because the polyp might be associated with anemia. Complete resection was achieved without any complications (Fig. 2). The final pathological diagnosis was Brunner’s gland hamartoma. After removal of the polyp, the patient’s anemia was improved and melena disappeared.
Brunner’s gland hamartoma is a rare benign duodenal tumor, and it accounts for less than 1% in primary tumors of the small intestine [1]. Although most cases were located at the duodenal bulb, it rarely originated in the stomach [1]. The cause of Brunner’s gland hamartoma remains unclear, but underlying risk factors have been suggested were high gastric acid secretion, H. pylori infection, chronic pancreatitis, inflammatory stimulation, and mucosal injury [1].
The clinical presentations are non-specific with various gastrointestinal symptoms including dyspepsia, abdominal distension, abdominal pain, bleeding, and obstruction [2,3]. Of the symptoms, gastrointestinal bleeding symptoms including melena and hematemesis were reported to be related to the polyp size [4]. In a study of 27 cases with Brunner’s gland hamartomas, the mean tumor size of patients developing gastrointestinal bleeding was 2.8 cm [5]. In the present case, the patient did not have a history of H. pylori eradication and showed a negative H. pylori infection on the pathological evaluation. The polyp size was 3.2×2.3 cm enough to cause gastrointestinal bleeding symptoms.
Most patients with Brunner’s gland hamartoma are asymptomatic, and endoscopic surveillance without resection is enough for small lesions. However, endoscopic resection of Brunner’s gland hamartoma is considered if patients have large tumor size (>10 mm), if patients present with bleeding or obstruction, or if patients have a suspicious feature of malignant potential such as an increase in the size of the polyp during a follow-up and subepithelial tumor-like lesion with central depression. The malignant potential of the Brunner’s gland hyperplasia lesions including hamartomas is low, and a retrospective study reported that dysplasia and invasive carcinoma were found in 2.1% and 0.3% of the 722 Brunner’s gland hyperplasia lesions, respectively [6]. The signs of potential malignant change of Brunner’s gland hamartoma included an increase in the size of the lesion with morphological change [1].
The present patient had a large tumor with gastrointestinal bleeding, and we removed Brunner’s gland hamartomas by EMR.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
REFERENCES
1. Zhu M, Li H, Wu Y, et al. Brunner’s gland hamartoma of the duodenum: a literature review. Adv Ther 2021;38:2779–2794.




2. Lee WC, Yang HW, Lee YJ, et al. Brunner’s gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy on pancreatic-duodenal area. J Korean Med Sci 2008;23:540–543.



3. Bojanapu S, Mangla V, Mehrotra S, Lalwani S, Mehta N, Nundy S. Brunner’s gland hyperplasia: an unusual duodenal submucosal lesion seen in four patients. J Surg Case Rep 2018;2018:rjy305.



4. Peloso A, Viganò J, Vanoli A, et al. Saving from unnecessary pancreaticoduodenectomy. Brunner’s gland hamartoma: case report on a rare duodenal lesion and exhaustive literature review. Ann Med Surg (Lond) 2017;17:43–49.


-
METRICS

-
- 0 Crossref
- 2,100 View
- 20 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Gastric Cancer of the Cardia Detected on Screening Endoscopy2023 September;23(3)
Bifurcated Lumen Observed on Capsule Endoscopy2023 September;23(3)
Tree-like Vessels on Magnifying Endoscopy2021 June;21(2)
Duodenal Carcinoid with Semipedunculated Polyp Treated by Endoscopic Resection2011 September;11(2)