Types of 23S Ribosomal RNA Point Mutations Affecting Helicobacter pylori Eradication Rates in Clarithromycin-Based Triple Therapy
Article information
Abstract
Objectives
The A2142G and A2143G mutations in the 23S ribosomal ribonucleic acid (rRNA) of Helicobacter pylori are the most common mutations associated with clarithromycin resistance. This study aimed to determine the differences in H. pylori eradication rates in patients infected with bacteria carrying the A2142G and A2143G mutations who were treated with clarithromycin-based triple therapy.
Methods
Data from a previous randomized controlled trial were analyzed retrospectively. Eradication rates were compared based on the presence of H. pylori carrying the A2142G and A2143G mutations. A meta-analysis was also conducted of relevant studies containing data regarding patients who received clarithromycin-based therapy due to infections with H. pylori harboring 23S rRNA mutations.
Results
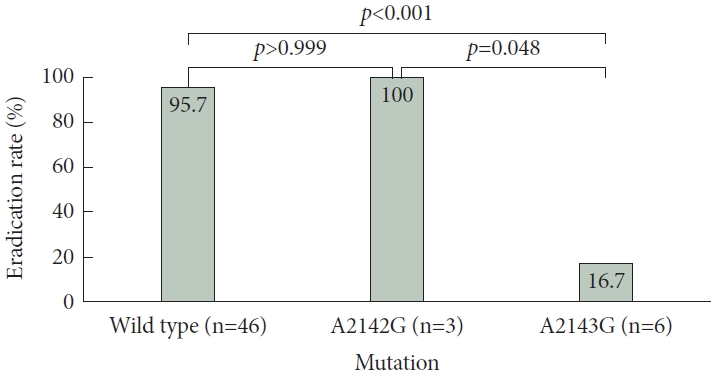
No significant difference was observed in H. pylori eradication rates between patients infected with wild-type bacteria (95.7% [44/46]) compared with those infected with bacteria carrying the A2142G mutation (100.0% [3/3]; p>0.9). However, the eradication rate was significantly lower for patients infected with bacteria carrying the A2143G mutation (16.7% [1/6]; p<0.001) than for those infected with wild-type bacteria or bacteria with the A2142G mutation (100.0% [3/3]; p=0.048). In the meta-analysis, the between-group comparisons yielded similar results. Although patients infected with bacteria having the A2142G mutation exhibited no significant risk difference (RD) for eradication compared with those infected with wild-type bacteria (RD=-0.05 [-0.18 to 0.08]; I2=0%; p=0.42), those infected with bacteria having the A2143G mutation demonstrated a lower H. pylori eradication rate compared with patients infected with either wild-type (RD=0.72 [0.64–0.80]; I2=0%; p<0.001) or A2143G mutant bacteria (RD=0.76 [0.61–0.91]; I2=0%; p< 0.001).
Conclusions
The A2143G mutation may play a more significant role in clarithromycin triple therapy H. pylori eradication failure than does the A2142G mutation. Additionally, H. pylori strains with the A2142G mutation can be treated effectively with clarithromycin-based triple therapy.
INTRODUCTION
Helicobacter pylori infection is the major cause of gastrointestinal diseases such as chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma [1-3]. Eradication of H. pylori prevents long-term complications and recurrence of gastrointestinal diseases caused by the infection [1,3-5]. Standard triple therapy (stan-dard-dose proton pump inhibitor [PPI], amoxicillin [1 g], and clarithromycin [500 mg] twice daily for 7 days) has been widely prescribed and recommended as a first-line treatment in Korea since 1998 [6]. However, the rate of clarithromycin resistance has increased continuously. A nationwide resistance study, conducted in 2018, reported a resistance rate of 17.8%–31% [7]. In the Maastricht V/Florence consensus report, therapies other than the standard triple therapy are recommended in areas with high (>15%) clarithromycin resistance rates [8]. According to the 2021 Korean guidelines, a clarithromycin resistance test determined using polymerase chain reaction (PCR) or sequencing is recommended when 7-day standard triple therapy is considered as a first-line treatment [6].
Point mutations in the peptidyl transferase region encoded in domain V of the H. pylori 23S ribosomal ribonucleic acid (rRNA) are crucial for clarithromycin resistance [9]. Several studies have demonstrated that among the point mutations known to cause clarithromycin resistance, the A2142G and A2143G mutations are the most common [9,10]. Among the available molecular tests, dual-priming oligonucleotide multiplex PCR (DPOPCR) has been commercialized and used in clinical practice and various clinical studies to detect A2142G and A2143G mutations. In multiple studies, a concordance rate of over 90% has been observed between DPO-PCR and culture-based susceptibility testing [11,12].
A randomized controlled trial (RCT) was conducted at Seoul National University Hospital from January 2019 to June 2019 to evaluate the efficacy of clarithromycin resistance-guided tailored therapy using the DPO-PCR test [13]. The present study aimed to retrospectively compare the effects associated with the A2142G and A2143G mutations on H. pylori eradication rates following clarithromycin-based triple therapy using a subgroup analysis of the previous RCT. A systematic literature review was also conducted to analyze relevant studies related to this topic.
METHODS
Trial design and patient selection for subgroup analysis
Among the patients in the previous RCT, those who were treated with clarithromycin-based triple therapy (esomeprazole [40 mg], amoxicillin [1 g], and clarithromycin [500 mg] twice daily for 10 days), regardless of the mutational status of the infecting H. pylori as determined by DPO-PCR, were selected for statistical analysis. As this study aimed to compare the contributory importance of each point mutation on the H. pylori eradication rate for clarithromycin-based triple therapy, the analysis was performed using the per-protocol method. All patients selected for subgroup analysis underwent a urea breath test (UBT) to confirm eradication. The eradication status was determined using 13C-UBT conducted at least 4 weeks after treatment completion. Patients were required to discontinue PPI or histamine-2 receptor blocker use for at least 2 weeks before the test.
Determination of clarithromycin resistance
Gastric biopsy specimens were obtained from the stomach antrum and body of each patient. Moreover, DPO-PCR was performed on the biopsy specimens using a commercial kit (Seeplex ClaR-H. pylori ACE Detection; Seegene Institute of Life Science, Seoul, Korea). The DPO-PCR results were interpreted as follows: wild-type bacteria were indicated by a single 621-bp deoxyribonucleic acid (DNA) product, bacteria harboring the A2142G mutation by a DNA band at 475 bp, and bacteria with the A2143G mutation by a DNA band at 194 bp. Detailed information about the PCR analysis is described in the previous study report [13].
Method for systematic search of relevant studies and meta-analysis
To find relevant studies, a systematic search was conducted on PubMed using the following keywords: “A2142G,” “A2143G,” “Helicobacter pylori,” and “Eradication.” Following a search using the aforementioned keywords, each article was reviewed to select studies containing data regarding patients infected with H. pylori strains carrying the A2142G or A2143G mutations and treated with clarithromycin-based triple therapy. Studies focusing solely on in vitro resistance profile tests, without eradication treatment, were excluded. Using this method, we aimed to identify studies specifically focusing on assessing the efficacy of clarithromycin-based triple therapy in patients infected with H. pylori strains carrying the A2142G and A2143G mutations. A meta-analysis was conducted to calculate pooled estimates of the eradication rates and to perform betweengroup comparisons for each mutation.
Statistical analysis
Fisher’s exact test and the chi-square test were used to compare categorical variables. The Wilcoxon rank-sum test was conducted to compare continuous variables exhibiting a nonnormal distribution. For the meta-analysis, a random-effects model was considered most appropriate because of the differing study designs, diverse study objectives, and variations in patient characteristics across the included studies. All statistical analyses were performed using R software, version 4.3.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria). The meta-analysis was performed using the “meta” package of the R software. Moreover, p-values less than 0.05 were considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Seoul National University Hospital, Seoul, Korea (2307-070-1448). The requirement for written consent was waived owing to the retrospective nature of this study which used data obtained from a previous RCT and a literature review of other relevant studies. This study was conducted following the principles of the Declaration of Helsinki.
RESULTS
Retrospective analysis of a previous RCT
Among the 72 patients enrolled in the previous RCT [13], nine were identified as being infected with H. pylori carrying either the A2142G or A2143G mutation; three were infected with H. pylori carrying the A2142G mutation, five with bacteria carrying the A2143G mutation, and one with bacteria carrying both mutations (classified into the A2143G mutation group, consistent with the study purpose and the analysis of other relevant studies [14-18]). These patients subsequently received clarithromycin-based triple therapy. The baseline characteristics of the patients included in the subgroup analyses are displayed in Table 1. No significant differences in the baseline characteristics were observed between the groups.
Additionally, no statistically significant differences were observed in the H. pylori eradication rates between patients affected by the wild-type bacteria and those infected with bacterial carrying A2142G mutation (95.7% [44/46] vs. 100.0% [3/3]; p>0.9). In contrast, patients infected with bacteria carrying the A2143G mutation exhibited a significantly lower eradication rate (16.7% [1/6]) than did those infected with wild-type bacteria (95.7% [44/46]; p<0.001) or those infected with bacteria carrying the A2142G mutation (100.0% [3/3]; p=0.048). A graphical representation of the results is illustrated in Fig. 1.
Literature search and meta-analysis of other related studies
A literature search was conducted using the aforementioned keywords, yielding a total of 77 articles. In most of these studies, patients infected with H. pylori strains carrying point mutations were not treated with clarithromycin-based therapy because of antibiotic resistance. After excluding studies that did not meet the prespecified criteria, five articles were identified. In three articles, the eradication rates were directly compared between patients infected with the A2142G and A2143G mutants. In the other two articles, although the primary goal was not to directly compare H. pylori eradication rates between groups of patients infected with the two mutant strains, subgroup analyses of the patient data were available.
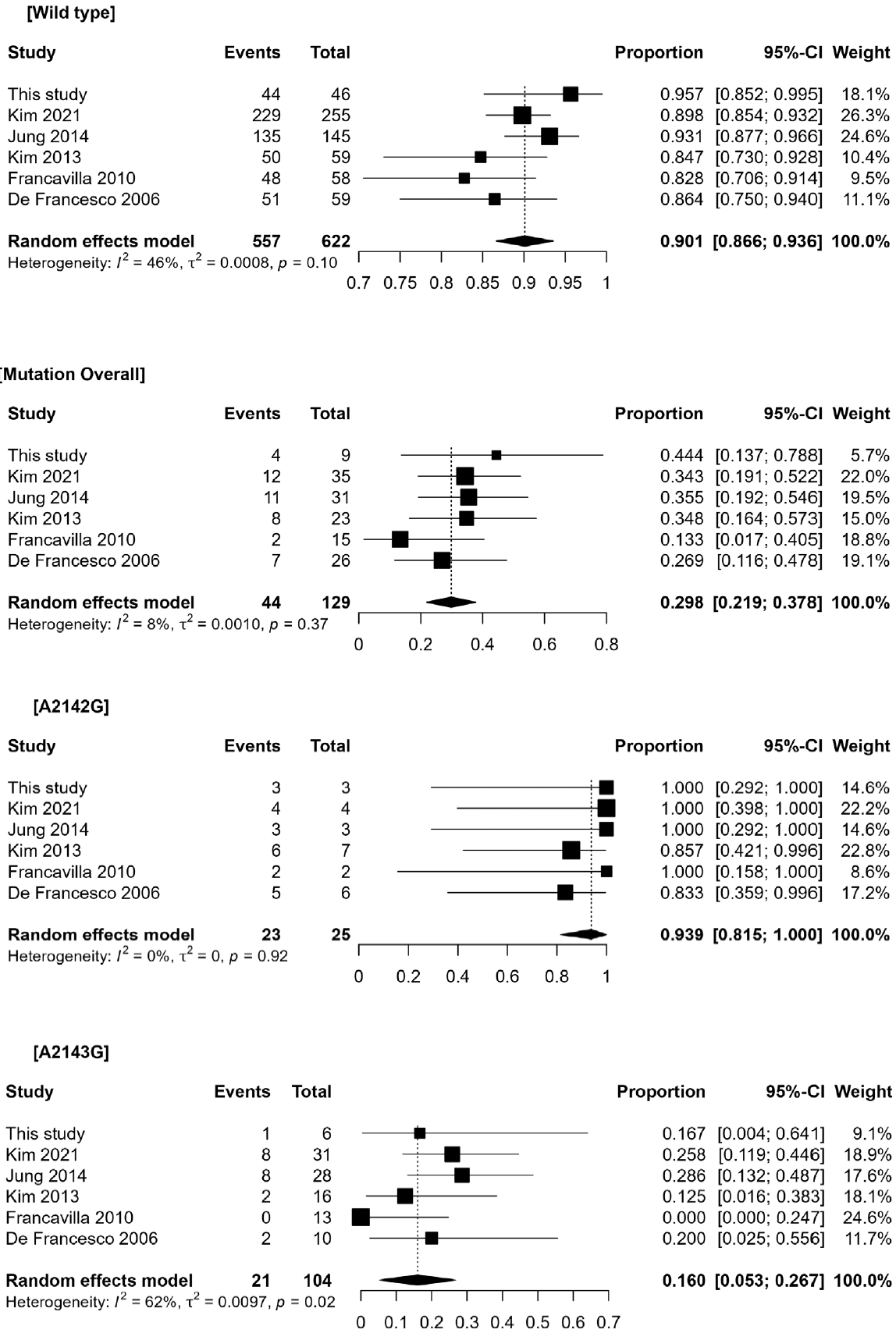
In a study conducted by Jung et al. [19], 145 patients were infected with wild-type H. pylori, 28 with bacteria carrying the A2143G mutation, and three with bacteria carrying the A2142G mutation. All patients were treated with standard triple therapy. The eradication rates did not differ between the patients infected with wild-type or A2142G mutant H. pylori (93.1% [135/145] vs. 100.0% [3/3], respectively; p>0.9). In contrast, the eradication rate associated with patients infected with A2143G mutants was significantly lower (28.5% [8/28]) than those of patients infected with A2142G mutants (100.0% [3/3]; p=0.036) or wild-type H. pylori (93.1% [135/145]; p<0.001).
In a study conducted by De Francesco et al. [15], 75 patients were assigned a 7-day standard triple therapy, including 59 patients infected with wild-type bacteria, 10 with bacteria having the A2143G mutation, and six with bacteria carrying the A2142G mutation. The H. pylori eradication rates were not different between patients infected with wild-type bacteria and those infected with A2142G mutants (86.4% [51/59] vs. 83.3% [5/6], respectively; p>0.9). In contrast, the eradication rate associated with patients infected with A2143G mutants (20.0% [2/10]) was significantly lower than those of individuals infected with either A2142G mutants (83.3% [5/6]; p=0.035) or wildtype bacteria (86.4% [51/59]; p<0.001).
Francavilla et al. [16] conducted a similar study using the same methodology as De Francesco et al. [15] In their study, 73 patients were assigned to the standard triple therapy group, including 58 infected with wild-type H. pylori, 13 with A2143G mutants, and two with A2142G mutants. The bacterial eradication rates were similar for patients infected with wild-type bacteria (82.8% [48/58]) and those infected with A2142G mutants (100.0% [2/2]; p>0.9). However, the eradication rate for patients infected with A2143G mutants (0.0% [0/13]) was significantly lower than the rate for patients infected with A2142G mutants (100.0% [2/2]; p=0.010) or wild-type H. pylori (82.8% [48/58]; p<0.001).
In a study by Kim et al. [17], 110 patients infected with H. pylori were treated with clarithromycin-based triple therapy. Information regarding the genotypic resistance profiles was obtained using DPO-PCR. Seven patients were infected with H. pylori carrying the A2142G mutation and 16 were infected with H. pylori carrying the A2143G mutation. No differences in bacterial eradication rates were observed between the patients infected with wild-type bacteria (84.7% [50/59]) and those infected with A2142G mutants (85.7% [6/7]; p>0.9). In contrast, the eradication rate for patients infected with A2143G mutants was significantly lower (12.5% [2/16]) than that for patients infected with either A2142G mutants (85.7% [6/7]; p=0.010) or wildtype bacteria (84.7% [50/59]; p<0.001).
In another study conducted by Kim et al. [18], 464 patients were retrospectively analyzed; 175 were infected with H. pylori harboring 23S rRNA mutations. Although the patients were assigned for treatment with bismuth-quadruple therapy based on their genotypic resistance status, 37 underwent clarithromycin-based triple therapy, despite the mutational status of the infecting bacteria; the therapy was prescribed by non-gastrointestinal specialists unfamiliar with the 23S rRNA mutation test. A subgroup analysis of those patients displayed no significant difference in the H. pylori eradication rates between the patients infected with wild-type bacteria (89.8% [229/255]) and those infected with A2142G mutants (100.0% [4/4]; p>0.9); a lower eradication rate was observed for patients in the A2143G H. pylori group (25.8% [8/31]) than in either the A2142G mutant (p=0.009) or wild-type H. pylori infection groups (p<0.001). A summary of the results of each study and the specifics of the eradication regimens used are provided in Table 2.

H.pylori eradication rate of clarithromycin-based triple therapy according to 23S rRNA point mutations
A meta-analysis of the six studies was performed using a random-effects model. Pooled analyses of the H. pylori eradication rates for each group yielded the following: 90.1% (95% confidence interval [CI], 86.6%–93.6%; I2=46%) for the wildtype bacterial infection group, 93.9% (95% CI, 81.5%–100%; I2=0%) for the A2142G mutant infection group, and 16.0% (95% CI, 5.3%–26.7%; I2=62%) for the A2143G mutant infection group. Between-group comparisons using the same statistical model yielded similar results. Although the patients infected with A2142G mutants exhibited no significant difference in their H. pylori eradication rate compared with those infected with wild-type bacteria (risk difference [RD]=-0.05 [95% CI, -0.18 to 0.08]; I2=0%; p=0.42), patients infected with A2143G mutants demonstrated a lower eradication rate compared with either those infected with wild-type bacteria (RD=0.72 [95% CI, 0.64–0.80]; I2=0%; p<0.001) or A2143G mutants (RD=0.76 [95% CI, 0.61–0.91]; I2=0%; p<0.001). The pooled analysis of the eradication rates and the between-group comparisons are displayed in Figs. 2 and 3, respectively.
DISCUSSION
This study demonstrated that, compared with the wild type, the A2143G mutation played a more crucial role in H. pylori eradication treatment failure than did the A2142G mutation. This result was consistent with other studies, including three studies conducted in Korea [17-19]. Among the various 23S rRNA point mutations identified from in vitro studies, the A2142G, A2142C, and A2143G mutations are commonly associated with high-level clarithromycin resistance. The T2182C, A2115G, G2141A, A2144G, and T2289C mutations have also been reported; however, their contribution to clarithromycin resistance is considered low, and their significance in the development of resistance is not fully understood [20,21]. Given the high prevalence of the A2142G, A2143G, and A2142C mutations and the high resistance rates associated with them, most of the commercialized kits designed to detect clarithromycin resistance related to 23S rRNA mutations involve tests targeting these mutations (Table 3) [9,22,23].
Although A2142G, A2143G, and A2142C are high-level resistance-related mutations, clinical studies that have specifically investigated the eradication rates of clarithromycin-based triple therapy involving these mutations in real-world settings are limited. Most studies have focused on the presence or absence of these mutations as predictive markers for clarithromycin resistance rather than directly comparing eradication rates among patients infected with H. pylori harboring the different mutations. In the present study, a direct statistical comparison of the clinical outcomes between mutations was performed. Considering the limitations of the small sample sizes, a review of other related studies was also conducted and analyzed. By including a comprehensive analysis of related studies, the A2143G mutation can be inferred to be a significant mutation contributing to eradication failure. The accumulated evidence from multiple studies strengthens this conclusion, despite the limitations of each study.
The mechanisms explaining the discrepancy between realworld treatment outcomes and in vitro studies regarding clarithromycin resistance are yet to be identified. Point mutations in domain V of the 23S rRNA can cause structural changes that hinder clarithromycin binding (the primary resistance mechanism) [20]. However, many other mechanisms also contribute to the development of resistance. One study demonstrated that different mutations in genes outside the 23S rRNA, such as rpl22 and infB, can mitigate clarithromycin resistance, even in the presence of 23S rRNA mutations [24]. Several studies have also revealed that the level of clarithromycin resistance in H. pylori strains carrying 23S rRNA mutation varies depending on the expression of efflux pumps [25,26]. Additionally, a recent study has demonstrated that bacterial biofilms are essential for the development of antibiotic resistance [27]. A study that utilized a computerized three-dimensional molecular structure prediction model demonstrated that 23S rRNA mutations hinder the binding of clarithromycin and induce secondary structural changes in other areas, including the nascent peptide exit tunnel, which is a crucial component of protein synthesis [28]. In an in vitro study, PPIs and amoxicillin have exerted synergistic effects with clarithromycin [29]. In addition to the possible causes mentioned above, various factors, including intragastric pH and several other host factors, are presumed to contribute to the differences in eradication rates. Future studies are needed to identify the reasons for the discrepancies between the findings of in vitro studies and the results of this analysis, including real-world clinical outcomes.
Most current guidelines recommend using non-clarithromycin-based therapies in regions where clarithromycin resistance is reported to be greater than 15% or when resistance is confirmed during testing [6,8]. However, this subgroup analysis and review of related studies suggested that standard clarithromycin triple therapy for 7 days remains a viable treatment option for patients with the A2142G mutation. Treatment regimens such as bismuth/metronidazole-based therapy have displayed excellent clinical outcomes for the treatment of clarithromycin-resistant strains [30,31]. However, a recent meta-analysis conducted in Korea exhibited a higher rate of adverse events and drug intolerance associated with quadruple therapy than with clarithromycin-based triple therapy [6]. Several studies have also reported increasing resistance rates to metronidazole and tetracycline, which could lower the eradication rate in the future [32,33]. Considering these factors, diversifying and preserving treatment options for H. pylori strains carrying the A2142G mutation may have clinical benefits.
Our study has certain limitations. First, the study was conducted retrospectively, which may have introduced a selection bias. Additionally, the analysis was performed using a per-protocol approach, including patients who were followed up and tested to confirm eradication, which may have overestimated the results. Second, although the differences in eradication rates were statistically significant, the sample sizes were small. However, statistical analyses were conducted on related studies, and the pooled results displayed findings similar to those of our analysis. When drawing conclusions from the meta-analysis, caution is necessary owing to the limited number of available studies, small sample sizes within each study, and the retro-spective design of all the included studies. Further research with larger patient cohorts and more robust study designs is warranted to better understand the effect of these mutations on treatment outcomes in clinical practice. These studies can provide valuable insights into the associations between specific 23S rRNA mutations and treatment success, ultimately leading to optimized treatment strategies for H. pylori eradication.
Conclusion
The eradication success rate was significantly lower in patients infected with H. pylori strains carrying the A2143G mutation than in those infected with wild-type bacteria or bacteria carrying the A2142G mutation. The eradication rate for strains with the A2142G mutation was not significantly different from that of the wild-type strain. Several other studies have displayed similar results, indicating the possibility of treating H. pylori strains carrying the A2142G mutation with standard 7-day clarithromycin triple therapy. These results also indicate the potential value of developing commercial kits that include crucial resistance-related point mutations other than the A2142G mutation in 23S rRNA.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Soo-Jeong Cho, a contributing editor of The Korean Journal of Helicobacter and Upper Gastrointestinal Research, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
None
Authors’ Contribution
Conceptualization: Soo-Jeong Cho. Data curation: Bokyung Kim, Hyunsoo Chung, Sang Gyun Kim, Soo-Jeong Cho. Formal analysis: Gihong Park. Investigation: Gihong Park. Methodology: Sang Gyun Kim, Soo-Jeong Cho. Project administration: Sang Gyun Kim, Soo-Jeong Cho. Resources: Soo-Jeong Cho. Software: Gihong Park. Supervision: Soo-Jeong Cho. Validation: Soo-Jeong Cho. Visualization: Gihong Park. Writing—original draft: Gihong Park. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Acknowledgements
None