 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 23(3); 2023 > Article |
|
See editorial "Scratch Sign as an Endoscopic Marker for Predicting the Absence of Current Helicobacter pylori Infection" in Volume 23 on page 157.
Abstract
Background/Aims
Identification of Helicobacter pylori infection status is necessary as H. pylori is associated with gastric malignancy. Recently, a red linear scrape-like appearance on the gastric mucosa, called the “scratch sign,” was reported to be associated with H. pylori-negative gastric mucosal status. Herein, we aimed to validate the association between the scratch sign and H. pylori infection status.
Methods
The data of patients who underwent screening endoscopy at Bundang Jesaeng General Hospital between March 2023 and April 2023 were reviewed. Patients were classified as having an H. pylori current infection or non-infection status based on the results of rapid urease tests. Patients who had undergone H. pylori eradication therapy were excluded. Endoscopic features of the gastric mucosa were assessed using the Kyoto classification of gastritis.
Results
The scratch sign appeared more frequently in patients with non-infection than in those with current infection status (32.7% vs. 10.6%, respectively; P<0.001). Multivariate analysis showed that only the presence of sticky mucus was significantly associated with the presence of the scratch sign. Patient without the scratch sign had a higher prevalence of open-type atrophy, intestinal metaplasia, enlarged folds, and diffuse redness, which reflected a higher Kyoto score.
Conclusions
Presence of the gastric mucosal scratch sign, a novel endoscopic marker, is indicative of H. pylori-negative status and appears to be inversely correlated with the presence of sticky mucus. In addition to the Kyoto classification of gastritis, detection of the scratch sign may facilitate identification of the H. pylori infection status.
Helicobacter pylori was first described in the 1980s, and the infection rate is currently greater than 50% worldwide [1,2]. Although the prevalence of H. pylori is declining, the World Health Organization and International Agency for Research on Cancer still classify H. pylori as a class 1 carcinogen and the primary cause of gastric cancer [3,4]. Eradication of H. pylori is known to be associated with a decreased risk of gastric cancer [5,6]. Therefore, it is important to predict and appropriately diagnose this infection early.
Recently, several attempts have been made to predict H. pylori infection status using various endoscopic features according to the Kyoto classification of gastritis [7]. The endoscopic findings associated with a current H. pylori infection status include atrophy, intestinal metaplasia, diffuse redness, spotty redness, edema, sticky mucus, enlarged folds, nodularity, xanthoma, foveolar hyperplastic polyps, and loss of regular arrangement of collecting venules (RAC). H. pylori-negative status includes fundic gland polyps, linear streaks, raised erosions, hematin, and the presence of RAC, and past infection status includes map-like redness, patchy redness, and multiple whitish flat lesions. Five representative endoscopic findings were scored to calculate the Kyoto classification score, with a score of 2 or higher suggesting the possibility of H. pylori infection [8]. Therefore, in addition to the conventional diagnostic method, these endoscopic findings have helped endoscopists to make a more accurate diagnosis of H. pylori.
During a typical upper endoscopic procedure, the duodenum is viewed first, followed by the stomach as the scope is slowly withdrawn. While observing the duodenum, the shaft of the endoscope passes over the greater curvature of the stomach and may cause a red linear scrape-like appearance with white deposit, which was named as “scratch sign” in a recent study [9]. The scratch sign is thought to occur due to mechanical stimulation related to the pressure of the scope and although this scratch sign is often seen in clinical practice, endoscopists have not attached any special significance to it. However, the study suggested that the scratch sign might be a good endoscopic predictor of H. pylori-negative gastric mucosa and found it to be useful in endoscopically diagnosing previously infected gastric mucosa when combined with atrophic changes and xanthomas that persisted after eradication of H. pylori [9,10]. Accordingly, we aimed to verify the association of the scratch sign combined with the Kyoto classification of gastritis with H. pylori status.
Patients who underwent screening endoscopy in the health care center of Bundang Jesaeng General Hospital between March 2023 and April 2023 were reviewed. Sex, age, underlying diseases, recent medication history, and history of eradication therapy were retrospectively investigated from electronic medical records. Patients with a history of previous H. pylori eradication treatment (n=65), taking acid suppression drugs (proton pump inhibitor [PPI], potassium-competitive acid blocker [PCAB], or H2 blocker) (n=43), diagnosed with advanced gastric cancer (n=1), gastric mucosa-associated lymphoid tissue lymphoma (n=1), and underwent subtotal gastrectomy (n=7) were excluded.
Patients were divided into H. pylori-positive status and H. pylori-negative status according to the results of a rapid urease test (RUT). This study was approved by the Ethics Review Board at Bundang Jesaeng General Hospital (approval number: 2023-04-005). Informed consent was waived by the IRB. All the research adhered to the ethical guidelines of the Declaration of Helsinki.
This study was retrospective single center study (Fig. 1). Initial endoscopic procedure was performed by 3 experienced endoscopists (JYS with 5 years of experience, HJC with 10 years of experience, and SJP with 25 years of experience), who were aware of the Kyoto classification of gastritis and scratch sign with GIF-H260 and GIF-H290 scopes (Olympus Medical Systems, Tokyo, Japan). The endoscopic procedure was performed with or without sedation using intravenous midazolam. After insertion of the scope orally, the second portion of the duodenum was initially assessed, and the gastric mucosa was evaluated in detail based on the Kyoto classification of gastritis. The presence of the scratch sign was assessed along the greater curvature (Fig. 2A). The presence of RAC was observed along the lesser curvature of the lower body (Fig. 2B). Atrophy was evaluated endoscopically based on the extent of atrophy and was categorized as non-atrophy, C1 to C3, or O1 to O3 as described by Kimura et al. [11,12]. We endoscopically evaluated intestinal metaplasia according to the extent of involvement at the antrum and corpus, using white light imaging and a villous appearance or whitish plaque was defined as endoscopic indicator of intestinal metaplasia. Gastric mucosal findings including fundic gland polyps (Fig. 2C), linear streaks, raised erosions (Fig. 2D), and hematin were classified as non-infected gastric mucosa. Other endoscopic findings including diffuse redness (Fig. 3A), spotty redness (Fig. 3B), edema, sticky mucus (Fig. 3C), enlarged folds, nodularity (Fig. 3D), xanthoma, and foveolar hyperplastic polyps were classified as currently infected gastric mucosa, while map-like redness, patchy redness, and multiple whitish flat lesions were classified as previously infected gastric mucosa. To reduce inter-observer variability, the endoscopists reviewed endoscopic images taken after the end of the examinations and reached a consensus regarding any ambiguous findings. All endoscopic images uploaded at the Picture Archive and Communication System (PACS) were retrospectively reviewed again by one endoscopist (JYS).
The Kyoto classification score is calculated based on five endoscopic features including atrophy, intestinal metaplasia, diffuse redness, enlarged folds, and nodularity and ranges from 0 to 8 points. The score for atrophy is based on the extent of atrophy with C-O or C-I being scored as 0 points, C-II or C-II as 1 point, and open-type atrophy (O-I, O-II, or O-III) as 2 points. For intestinal metaplasia, which is assessed using white-light endoscopy, a score of 0 is given if there is no metaplasia, 1 if it is limited to the antrum, and 2 if it has spread to the corpus. Diffuse redness, which is indicative of overall reddish mucosal changes, is observed in non-atrophic mucosa, mild diffuse redness with RAC is scored as 1, and severe diffuse redness without RAC is scored as 2. Enlarged folds are defined as thickened gastric folds measuring more than 5 mm in size before air inflation and are scored as 1 point if present. Nodularity, which indicates a nodular mucosal appearance resembling goose skin, is scored as 1. The total Kyoto classification score is the sum of the scores for these five features.
To determine the presence of H. pylori infection, we conducted a RUT (Helicobacter test; In Fung, Taichung City, Taiwan). The RUT involves obtaining gastric mucosal tissue from the atrophy-free portion of the antrum and corpus. We defined H. pylori-positive status as a positive RUT and H. pylori-negative status as negative results on the RUT.
The data were analyzed using R version 4.2.2 (R Foundation for statistical Computing, Vienna, Austria). Continuous variables were presented as mean with standard deviation, while categorical variables are presented as percentages. The relationship between two categorical variables was determined using the chi-square test. Univariate and multivariate logistic regression analyses were conducted to predict the likelihood of H. pylori infection and the presence of the scratch sign based on various endoscopic findings, and the results were reported as odds ratios (ORs) along with corresponding P-values. A P-value less than or equal to 0.05 was considered statistically significant.
The number of 376 patients with H. pylori non-infection and 226 patients with H. pylori infection were included. While the sex ratio in infected patients was 50%, but in non-infected patients, there was significantly higher proportion of male (232/144 vs. 113/113, P=0.006). None of the other characteristics showed significant differences between these two groups (Table 1). When comparing the groups with and without the scratch sign, there were no significant in baseline characteristics (Supplementary Table 1).
The rate of a scratch sign was significantly higher in patients of non-infection than in those with a current infection (32.7% vs. 10.6%, P<0.001). Non-infected patients also had a significantly higher rate of RAC (33.8% vs. 1.8%, P<0.001), fundic gland polyps (12.0% vs. 1.8%, P<0.001), linear streaks (48.1% vs. 7.1%, P<0.001), raised erosions (42.3% vs. 21.7%, P<0.001), hematin (32.7% vs. 10.6%, P<0.001), and closed type atrophy (38.8% vs. 20.4%, P<0.001). In H. pylori-infected patients, there were significantly higher rates of open-type atrophy (77.0% vs. 25.8%), intestinal metaplasia confined to antrum (9.3% vs. 5.1%) or corpus (61.9% vs. 23.9%), diffuse redness (78.3% vs. 6.1%, P<0.001), spotty redness (67.3% vs. 6.4%, P<0.001), edema (62.4% vs. 13.0%, P<0.001), sticky mucus (38.5% vs. 1.9%, P<0.001), enlarged folds (27.9% vs. 2.9%, P<0.001), nodularity (21.2% vs. 1.1%, P<0.001), xanthoma (8.8% vs. 4.0%, P=0.022), and hyperplastic polyp (3.5% vs. 0.8%, P=0.001) than in non-infected patients (Table 2).
A total of 147 patients had the scratch sign. These patients had a significantly higher rate of RAC (36.1% vs. 17.1%, P<0.001), fundic gland polyps (12.9% vs. 6.6%, P=0.023), and linear streaks (46.3% vs. 28.4%, P<0.001) than those without the scratch sign. In contrast, open-type atrophy (25.2% vs. 51.4%, P<0.001), intestinal metaplasia confined to antrum (6.1% vs. 6.8%, P<0.001) or corpus (23.1% vs. 43.1%, P<0.001), diffuse redness (11.6% vs. 40.2%, P<0.001), spotty redness (9.5% vs. 35.6%, P<0.001), edema (15.0% vs. 36.9%, P<0.001), enlarged folds (0.7% vs. 16.0%, P<0.001), and sticky mucus (0.7% vs. 20.4%, P<0.001) were significantly less common in those with the scratch sign. Among the patients with the sticky mucus and no scratch sign, 86 of 94 patients (91.5%) had a current H. pylori infection. There was a significant difference in the Kyoto score (1.7±1.9 vs. 3.3±2.5, P<0.001) between the groups (Table 3).
In the univariate logistic analysis for predicting H. pylori infection, all variables including the scratch sign (OR 0.24, 95% confidence interval [CI] 0.15~0.39, P<0.001) showed a significant association with H. pylori infection. However, in the multivariate analysis, linear streak (OR 0.33, 95% CI 0.15~0.71, P=0.006), and hematin (OR 0.37, 95% CI 0.17~0.75, P=0.007) were found to be associated with a decreased risk of H. pylori infection, while diffuse redness (OR 10.64, 95% CI 5.40~21.61, P<0.001), spotty redness (OR 2.83, 95% CI 1.34~5.96, P=0.006), edema (OR 3.28, 95% CI 1.75~6.20, P<0.001), sticky mucus (OR 6.75, 95% CI 2.48~21.19, P<0.001), and nodularity (OR 7.00, 95% CI 2.12~28.70, P=0.003) were associated with an increased risk of H. pylori infection (Table 4).
In the univariate logistic regression analysis for the presence of scratch sign, several endoscopic features such as the presence of RAC, fundic gland polyps, linear streaks, atrophy, intestinal metaplasia, diffuse redness, spotty redness, edema, sticky mucus, and enlarged folds were found to be associated with the scratch sign. However, the multivariate analysis showed that the presence of sticky mucus was associated with significantly fewer scratch signs (OR 0.10, 95% CI 0.01~0.49, P=0.025); other factors did not show significant associations (Table 5).
Recently, several studies have investigated endoscopic features as potential predictors of H. pylori infection status, and the emergence of the Kyoto classification of gastritis has facilitated this process [13-15]. In addition, a recent study reported that the scratch sign reflects H. pylori non-infection status [9]. Therefore, we sought to validate the utility of the scratch sign as a marker of H. pylori-negative status in patients undergoing screening endoscopy in health care center.
The scratch sign is a visible feature that occurs during endoscopy when the shaft of the endoscope scrapes against the greater curvature of the body of the stomach. It is thought to occur due to mechanical stimulation related to the pressure of the scope. On detailed inspection, when the shaft of the scope passes through and denudes the mucosal layer, the vascular architecture of the lamina propria is exposed. A previous study suggested that this is related to the presence of a mucus layer [9]. In mucosa not infected by H. pylori, the mucus gel layer is preserved. However, when severe atrophy and intestinal metaplasia occur due to infection or when acid suppression medications such as PPIs or PCABs are taken, the mucus gel layer diminishes [16-18]. Our study also showed that the degree of atrophy (open-type atrophy) and intestinal metaplasia were more severe in the absence of the scratch sign (Table 3), which was consistent with the findings of previous studies.
While previous study has showed significant differences in gender and medication use between patients with and without the scratch sign, our study showed that there was no significant characteristics between the groups (Supplementary Table 1) [9]. In our study, we used RUT to evaluate H. pylori status, so we excluded the patients taking acid suppression medication (PPI, PCAB, and H2 blocker) to rule out false negative result. Therefore, it would be needed to analyze the association between the medication and endoscopic findings in the further study.
Our study confirmed that the Kyoto classification of gastritis accurately reflected H. pylori status based on detailed examination of the gastric mucosa in our health screening patients. However, due to the limited number of patients who was treated with eradication treatment among those who visited the healthcare center (n=65), we were unable to investigate previously infected mucosal lesions. Future studies are needed to investigate the presence of the scratch sign in patients with prior infections and naive patients.
Yada et al. [9] previously reported a significant association between a negative scratch sign and higher degrees of mucosal lesions, such as atrophy, intestinal metaplasia, enlarged folds, and diffuse redness, which all contribute to the Kyoto score. In our study, we also observed significant differences in the Kyoto score between patients with and without the scratch sign (1.7 vs. 3.3, P<0.001), reflecting similar findings. These finding would suggest that the absence of the scratch sign may indicate a higher possibility of current infection because of higher Kyoto score.
In a previous study, the scratch sign had a high specificity and positive predictive value for indicating H. pylori-negative status, as only one patient was infected in the group with the scratch sign [9]. However, in our study, we found the number of 24 patients with current infections had scratch sign (Table 2) and patients with scratch sign had significantly lower rate of sticky mucus (0.7% vs. 20.4%, P<0.001). These findings were also shown in multivariate analysis, which found the only sticky mucus was significantly lowered the risk of scratch sign (Table 5). This might be because the presence of sticky mucus could act as a lubricant, preventing the shaft of the endoscope from creating the scratch sign as it enters the duodenum.
There are a few limitations in our study that should be considered. First, we relied on patient’s history of prior H. pylori eradication to distinguish between prior H. pylori non-infection and past infection. It would have been ideal to distinguish between past infection and naive patients using the pepsinogen test, but unfortunately, we were unable to obtain the pepsinogen test at our center. Second, as this was a retrospective single center study and the endoscopic images were reviewed by a single endoscopist, inter-observer variation may have occurred. Therefore, further research will be needed to validate these endoscopic finding in multicenter studies.
In conclusion, our study found that the scratch sign is a useful endoscopic marker for identifying patients with H. pylori-negative status, and we observed an association with the presence of sticky mucus. In addition to the Kyoto classification of gastritis, these findings may help endoscopists to identify H. pylori infection status.
Notes
AVAILABILITY OF DATA AND MATERIAL
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Jun-young Seo. Data curation: Jun-young Seo, Hyo-Jin Cho, and Sang Jong Park. Formal analysis: Jun-young Seo. Investigation: Jun-young Seo. Methodology: Jun-young Seo. Writing - original draft: Jun-young Seo. Writing - review & editing: Jun-young Seo, Sang Jong Park, Ah Young Lee, Sang-Jung Kim, and Ji Yong Ahn.
Acknowledgements
We thank our gastroenterology fellows (Hyun Tak Lee, Boram Seo, Jeong Hwan Lee, and Jeong Woo Lee) for the description of endoscopic findings.
Fig. 2.
Endoscopic images of signs that indicate Helicobacter pylori non-infection status. (A) Scratch sign. (B) Regular arrangement of collecting venules. (C) Fundic gland polyp. (D) Raised erosions.
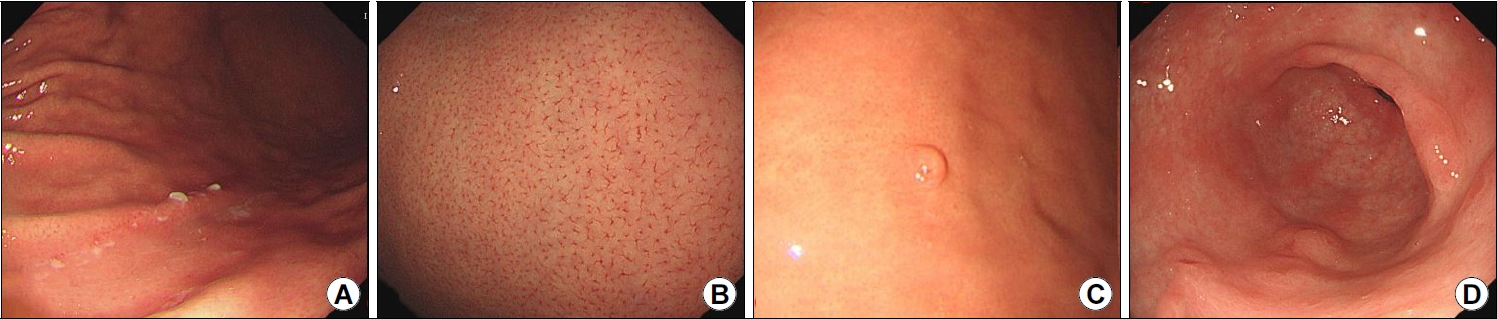
Fig. 3.
Endoscopic images of signs that indicate Helicobacter pylori infection status. (A) Spotty redness. (B) Diffuse redness. (C) Sticky mucus. (D) Nodularity.

Table 1.
Baseline Characteristics of Patients with and without Helicobacter pylori Infection
Table 2.
Endoscopic Findings of Patients with Helicobacter pylori Infection and Non-infection Statuses
| Variable | Non-infection (n=376) | Current infection (n=226) | P-value | ||
|---|---|---|---|---|---|
| Negative findings | |||||
| RAC | 127 (33.8) | 4 (1.8) | <0.001 | ||
| Fundic gland polyp | 45 (12.0) | 4 (1.8) | <0.001 | ||
| Linear Streak | 181 (48.1) | 16 (7.1) | <0.001 | ||
| Raised erosion | 159 (42.3) | 49 (21.7) | <0.001 | ||
| Hematin | 145 (32.7) | 24 (10.6) | <0.001 | ||
| Scratch sign | 123 (32.7) | 24 (10.6) | <0.001 | ||
| Positive findings | |||||
| Atrophya | <0.001 | ||||
| Absent | 133 (35.4) | 6 (2.7) | |||
| Closed type | 146 (38.8) | 46 (20.4) | |||
| Open type | 97 (25.8) | 174 (77.0) | |||
| Intestinal metaplasia | <0.001 | ||||
| Antrum | 19 (5.1) | 21 (9.3) | |||
| Corpus | 90 (23.9) | 140 (61.9) | |||
| Diffuse redness | 23 (6.1) | 177 (78.3) | <0.001 | ||
| Spotty redness | 24 (6.4) | 152 (67.3) | <0.001 | ||
| Edema | 49 (13.0) | 141 (62.4) | <0.001 | ||
| Sticky mucus | 7 (1.9) | 87 (38.5) | <0.001 | ||
| Enlarged folds | 11 (2.9) | 63 (27.9) | <0.001 | ||
| Nodularity | 4 (1.1) | 48 (21.2) | <0.001 | ||
| Xanthoma | 15 (4.0) | 20 (8.8) | 0.022 | ||
| Hyperplastic polyp | 3 (0.8) | 8 (3.5) | 0.034 | ||
| Past-infection findings | |||||
| Map-like redness | 12 (3.2) | 3 (1.3) | 0.250 | ||
| Multiple whitish lesions | 27 (7.2) | 5 (2.2) | 0.015 | ||
| Kyoto scores | 1.6±1.7 | 5.1±1.8 | <0.001 | ||
Table 3.
Endoscopic Findings of the Study Patients according to the Presence of the Scratch Sign
| Variable | Negative scratch sign (n=455) | Positive scratch sign (n=147) | P-value | ||
|---|---|---|---|---|---|
| Negative findings | |||||
| RAC | 78 (17.1) | 53 (36.1) | <0.001 | ||
| Fundic gland polyp | 30 (6.6) | 19 (12.9) | 0.023 | ||
| Linear Streak | 129 (28.4) | 68 (46.3) | <0.001 | ||
| Raised erosion | 158 (34.7) | 50 (34.0) | 0.954 | ||
| Hematin | 121 (26.6) | 47 (32.0) | 0.247 | ||
| Positive findings | |||||
| Atrophya | <0.001 | ||||
| Absent | 81 (17.8) | 58 (39.5) | |||
| Closed type | 140 (30.8) | 52 (35.4) | |||
| Open type | 234 (51.4) | 37 (25.2) | |||
| Intestinal metaplasia | <0.001 | ||||
| Antrum | 31 (6.8) | 9 (6.1) | |||
| Corpus | 196 (43.1) | 34 (23.1) | |||
| Diffuse redness | 183 (40.2) | 17 (11.6) | <0.001 | ||
| Spotty redness | 162 (35.6) | 14 (9.5) | <0.001 | ||
| Edema | 168 (36.9) | 22 (15.0) | <0.001 | ||
| Sticky mucus | 93 (20.4) | 1 (0.7) | <0.001 | ||
| Enlarged folds | 73 (16.0) | 1 (0.7) | <0.001 | ||
| Nodularity | 41 (9.0) | 11 (7.5) | 0.686 | ||
| Xanthoma | 33 (7.3) | 2 (1.4) | 0.014 | ||
| Hyperplastic polyp | 11 (2.4) | 0 (0.0) | 0.122 | ||
| Past-infection findings | |||||
| Map-like redness | 13 (2.9) | 2 (1.4) | 0.479 | ||
| Multiple whitish lesions | 26 (5.7) | 6 (4.1) | 0.578 | ||
| Kyoto scores | 3.3±2.5 | 1.7±1.9 | <0.001 | ||
Table 4.
Univariate and Multivariate Logistic Analysis of Endoscopic Findings Associated with Helicobacter pylori Infection
Table 5.
Univariate and Multivariate Logistic Analysis of Endoscopic Findings Associated with the Scratch Sign
REFERENCES
1. Kamboj AK, Cotter TG, Oxentenko AS. Helicobacter pylori: the past, present, and future in management. Mayo Clin Proc 2017;92:599-604.

2. Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 2012;17 Suppl 1:1-8.


4. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 1994;61:1-241.


5. Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016;150:1113-1124; e5.


6. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and metaanalysis. Gut 2020;69:2113-2121.


7. Kamada T, Haruma K, Inoue K, Shiotani A. [Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis]. Nihon Shokakibyo Gakkai Zasshi 2015;112:982-993; Japanese.

8. Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol 2020;26:466-477.



9. Yada T, Itakura Y, Watanabe R, et al. A novel endoscopic finding of a scratch sign is useful for evaluating the Helicobacter pylori infection status. DEN Open 2022;3:e200.




10. Watanabe K, Nagata N, Shimbo T, et al. Accuracy of endoscopic diagnosis of Helicobacter pylori infection according to level of endoscopic experience and the effect of training. BMC Gastroenterol 2013;13:128.




11. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969;1:87-97.

12. Toyoshima O, Yamaji Y, Yoshida S, et al. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc 2017;31:2140-2148.




13. Haruma K, Kato M, Inoue K, Murakami K, Kamada T. Kyoto classification of gastritis. Tokyo: Nihon Medical Center, 2017.
14. Yoshii S, Mabe K, Watano K, et al. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc 2020;32:74-83.



15. Hirai R, Hirai M, Shimodate Y, et al. Feasibility of endoscopic evaluation of Helicobacter pylori infection status by using the Kyoto classification of gastritis in the population-based gastric cancer screening program: a prospective cohort study. Health Sci Rep 2021;4:e325.




16. Bansil R, Celli JP, Hardcastle JM, Turner BS. The influence of mucus microstructure and rheology in Helicobacter pylori infection. Front Immunol 2013;4:310.



17. Matsuzaki J, Suzuki H, Minegishi Y, et al. Acid suppression by proton pump inhibitors enhances aquaporin-4 and KCNQ1 expression in gastric fundic parietal cells in mouse. Dig Dis Sci 2010;55:3339-3348.



18. Celli JP, Turner BS, Afdhal NH, et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci U S A 2009;106:14321-14326.



19. Kohli Y, Kato T, Suzuki K, Tada T, Fujiki N. Incidence of atrophic gastritis with age in Japan and Canada. Jpn J Med 1987;26:158-161.


- TOOLS








