 |
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 19(2); 2019 > Article |
|
Abstract
Gut microbiota have been known to play an essential role in host immunity and metabolism. Dysbiosis is associated with various gastrointestinal (GI) and other diseases such as cancers, metabolic diseases, allergies, and immunological disorders. So far, the role of gut microbiota has been studied mainly in lower GI disease but has recently been reported in upper GI diseases other than Helicobacter pylori infection, including BarrettŌĆÖs esophagus, esophageal carcinoma, gastric cancer, functional dyspepsia, and nonsteroidal anti-inflammatory drug-induced small intestinal mucosal injury. Probiotics have some beneficial effect on these diseases, but the effects are strain specific.
ņןļé┤ ņäĖĻĘĀņ┤ØņØĆ ņØĖĻ░äņØś ļīĆņé¼ ĻĖ░ļŖźĻ│╝ ļ®┤ņŚŁ ĻĖ░ļŖźņŚÉ ņżæņÜöĒĢ£ ņŚŁĒĢĀņØä ĒĢśļ®░, ņןļé┤ ņäĖĻĘĀņ┤ØņØś ņØ┤ņāüņØĆ ņŚ╝ņ”Øņä▒ ņןņ¦łĒÖśņØ┤ļéś Ļ│╝ļ»╝ņä▒ ņןņ”ØĒøäĻĄ░Ļ│╝ Ļ░ÖņØĆ ņåīĒÖöĻĖ░ ņ¦łĒÖś ņØ┤ņÖĖņŚÉļÅä ļ╣äļ¦ī, ļŗ╣ļć©, ņŚ╝ņ”Ø ļ░Å ļīĆņé¼ņä▒ ņ¦łĒÖś, ņ×ÉĻ░Ćļ®┤ņŚŁ ņ¦łĒÖś, Ē¢ēļÅÖ ņØ┤ņāü, ļÅÖļ¦źĻ▓ĮĒÖöņ”Ø ļō▒ņØś ļŗżņ¢æĒĢ£ ņ¦łĒÖśļōżĻ│╝ ņŚ░Ļ┤ĆļÉ£ļŗż[1]. ņāüļČĆņ£äņןĻ┤Ć ņ¦łĒÖś ņżæņŚÉņä£ļŖö Helicobacter pylori (H. pylori) Ļ░ÉņŚ╝ņØ┤ ņ£äņŚ╝ ļ░Å ņ£äņĢö ļ░£ņāØņŚÉ Ļ┤ĆņŚ¼ĒĢśļŖö ĻĖ░ņĀäņØ┤ ņל ņĢīļĀżņĀĖ ņ׳ņ£╝ļ®░ ņ£ä ņäĖĻĘĀņ┤ØņØś ņØ┤ņāüņØ┤ ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”ØĻ│╝ ņŚ░Ļ┤ĆļÉ£ļŗżļŖö ņŚ░ĻĄ¼ļÅä ņ׳ļŗż[2,3]. ļśÉĒĢ£ ļ░öļĀøņŗØļÅäņÖĆ ļ╣äņŖżĒģīļĪ£ņØ┤ļō£ņåīņŚ╝ņĀ£(nonsteroidal anti-inflammatory drug, NSAID)ņŚÉ ņØśĒĢ£ ņåīņן ņĀÉļ¦ē ņåÉņāü ļ░£ņāØ ĻĖ░ņĀäņŚÉļÅä ņןļé┤ ņäĖĻĘĀņ┤ØņØ┤ Ļ┤ĆņŚ¼ĒĢ£ļŗż(Fig. 1) [4,5]. ņØ┤ļ¤¼ĒĢ£ ņןļé┤ ņäĖĻĘĀņ┤Ø ņØ┤ņāüĻ│╝ ņŚ░Ļ┤ĆļÉśļŖö ņ¦łĒÖśņØś ņ╣śļŻī ļ░®ļ▓Ģ ņżæ ĒĢśļéśļĪ£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖż(probiotics)Ļ░Ć ņé¼ņÜ®ļÉĀ ņłś ņ׳ļŗż. ļ│ĖĻ│ĀņŚÉņä£ļŖö ņāüļČĆņ£äņןĻ┤Ć ņ¦łĒÖśņŚÉņä£ ņןļé┤ ņäĖĻĘĀņ┤ØņØś ņŚŁĒĢĀĻ│╝ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżņØś ņĀüņÜ®ņŚÉ ļīĆĒĢśņŚ¼ ņé┤ĒÄ┤ļ│┤Ļ│Āņ×É ĒĢ£ļŗż.
ņØĖĻ░äņØś ĻĄ¼Ļ░ĢļČĆĒä░ ļīĆņןĻ╣īņ¦Ć ļ¬©ļōĀ ņåīĒÖöĻ┤ĆņŚÉ ņäĖĻĘĀņØ┤ ņĪ┤ņ×¼ĒĢśņ¦Ćļ¦ī ĻĘĖ ļīĆļČĆļČäņØĆ ļīĆņןņŚÉ ņ׳ĻĖ░ ļĢīļ¼ĖņŚÉ ņןļé┤ ņäĖĻĘĀņ┤Ø ņŚ░ĻĄ¼ļŖö ņŻ╝ļĪ£ ļīĆļ│ĆņØä ņØ┤ņÜ®ĒĢśņŚ¼ ņ¦äĒ¢ēļÉśĻ│Ā ņ׳ļŗż. ņØĖĻ░äņØś ņןļé┤ ņäĖĻĘĀņØĆ ņ▓┤ņäĖĒżņłśļ│┤ļŗż ņĢĮ 10ļ░░ ļ¦ÄņØĆ Ļ▓āņ£╝ļĪ£ ņĢīļĀżņĀĖ ņ׳ņ£╝ļéś, ņØ┤ ņł½ņ×ÉļŖö ņśżļלļÉ£ ļģ╝ļ¼ĖņØś ļīĆļץņĀüņØĖ Ļ│äņé░ Ļ▓░Ļ│╝ņŚÉ ĻĘ╝Ļ▒░ĒĢśĻ│Ā ņ׳ļŗż[6]. ņĄ£ĻĘ╝ ņāłļĪ£ņÜ┤ Ļ│äņé░ ļ░®ļ▓ĢņØä ņØ┤ņÜ®ĒĢ£ ņŚ░ĻĄ¼ņŚÉņä£ ņ▓┤ņäĖĒżņłśņÖĆ ņןļé┤ ņäĖĻĘĀņłśĻ░Ć 1:1ņŚÉ ĻĘ╝ņĀæĒĢśņŚ¼ ļé©ņ×ÉņØś Ļ▓ĮņÜ░ 1.3:1 (38├Ś1012:30├Ś1012), ņŚ¼ņ×ÉņØś Ļ▓ĮņÜ░ 2.2:1 (44├Ś1012:21├Ś1012)ļĪ£ Ļ│äņé░ļÉśņŚłļŗż[7]. ņĄ£ņŗĀ ĻĖ░ļ▓ĢņØĖ 16S rRNA ņ░©ņäĖļīĆ ņŚ╝ĻĖ░ ļČäņäØ(next generation sequencing) ļ░®ļ▓Ģņ£╝ļĪ£ Ļ▓ĆņČ£ļÉ£ ņןļé┤ ņäĖĻĘĀņØś ņĢĮ 80%ļŖö ļ░░ņ¢æņØ┤ ļČłĻ░ĆļŖźĒĢśĻ▒░ļéś ņĢäņ¦üĻ╣īņ¦Ć ļ░░ņ¢æņØ┤ ļÉśņ¦Ć ņĢŖņØĆ ĻĘĀņØ┤ļŗż[8]. ņØĖĻ░äņØś ļŗ©ļ░▒ņ¦ł ņĮöļö® ņ£ĀņĀäņ×ÉļŖö ņĢĮ 20,000Ļ░£ņŚÉ ļČłĻ│╝ĒĢśņ¦Ćļ¦ī ņןļé┤ ņäĖĻĘĀņ┤ØņØś ļŗ©ļ░▒ ņĮöļö® ņ£ĀņĀäņ×ÉļŖö ņĢĮ 100,000Ļ░£ļĪ£ ņØĖĻ░äņØś ļīĆņé¼ ĻĖ░ļŖź ņżæ ļ¦ÄņØĆ ļČĆļČäņØä ņןļé┤ ņäĖĻĘĀņ┤ØņØ┤ ļŗ┤ļŗ╣ĒĢśļŖö Ļ▓āņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż[9]. ļ¼┤ĻĘĀ ņāüĒā£ņØś Ēā£ņĢäĻ░Ć ņé░ļ¬©ņØś ņé░ļÅäļź╝ ĒåĄĒĢśņŚ¼ ņČ£ņāØĒĢśļŖö Ļ│╝ņĀĢņŚÉņä£ ņןļé┤ ņäĖĻĘĀņØś ņĀĢņ░®ņØ┤ ņŗ£ņ×æļÉśļŖöļŹ░, ņĀ£ņÖĢņĀłĻ░£ļź╝ ĒåĄĒĢśņŚ¼ Ēā£ņ¢┤ļéśļŖö ņŗĀņāØņĢäņÖĆ ņ¦łņŗØ ļČäļ¦īņ£╝ļĪ£ Ēā£ņ¢┤ļé£ ņŗĀņāØņĢäļŖö ņןļé┤ ņäĖĻĘĀļōżņØ┤ ņĀĢņ░®ļÉśļŖö ņł£ņä£Ļ░Ć ļŗżļź┤ļŗż. ņןļé┤ ņäĖĻĘĀņ┤ØņØĆ ņĢĮ 3ņäĖĻ▓ĮņŚÉ ņä▒ņØĖĻ│╝ ņ£Āņé¼ĒĢ£ ņłśņżĆņ£╝ļĪ£ ļ░£ļŗ¼ĒĢśĻ▓ī ļÉśņ¢┤ ņä▒ņØĖĻĖ░ņŚÉļŖö ņØ╝ņĀĢĒĢśĻ▓ī ņ£Āņ¦ĆļÉśĻ│Ā, ņ£äņןĻ┤Ć ņāØļ”¼ĻĖ░ļŖź ņĀĆĒĢśĻ░Ć ļéśĒāĆļéśļŖö 70ņäĖ ņØ┤ņāüņØś Ļ│ĀļĀ╣ņØ┤ ļÉśļ®┤ņä£ ņןļé┤ ņäĖĻĘĀņ┤ØņØś ņĪ░ņä▒ļÅä ļ│ĆĒÖöļÉśĻĖ░ ņŗ£ņ×æĒĢ£ļŗż. ņןļé┤ ņäĖĻĘĀņ┤ØņØś ņĪ░ņä▒ņØĆ ņØīņŗØ, ĒÖśĻ▓Į, ĒĢŁņāØņĀ£, ņĢĮņĀ£ ļō▒ ņŚ¼ļ¤¼ ņÜöņØĖņŚÉ ņśüĒ¢źņØä ļ░øņĢä ņØ╝ņŗ£ņĀüņ£╝ļĪ£ ļ│ĆĒĢĀ ņłś ņ׳ļŖöļŹ░[10], ļ¦īņĢĮ ņØ┤ļ¤░ ļ│ĆĒÖöĻ░Ć ĒÜīļ│ĄļÉśĻĖ░ ņ¢┤ļĀżņÜĖ ņĀĢļÅäļĪ£ ļäłļ¼┤ ņŗ¼ĒĢśĻ▒░ļéś ņśżļל ņ¦ĆņåŹļÉśļ®┤ ņןļé┤ ņäĖĻĘĀņ┤ØņØś ĻĘĀĒśĢņØ┤ Ļ╣©ņ¦ĆĻ▓ī ļÉśņ¢┤ ņןļé┤ ņäĖĻĘĀ ļČłĻĘĀĒśĢ(dysbiosis)ņØ┤ ļ░£ņāØĒĢśĻ▓ī ļÉ£ļŗż[11].
ņŗØļÅäļŖö ņŗØļÅä ņāüņ×¼ĻĘĀ, ĻĄ¼Ļ░ĢņŚÉņä£ ļäśņ¢┤ņś© ĻĘĀ ļśÉļŖö ņ£äņŚÉņä£ ņŚŁļźśļÉ£ ĻĘĀņŚÉ ļģĖņČ£ļÉ£ļŗż. ņŚŁļźśņä▒ ņŗØļÅäņŚ╝Ļ│╝ ļ░öļĀøņŗØļÅäņÖĆ Ļ░ÖņØ┤ ļ¦īņä▒ņĀüņØĖ ņŚ╝ņ”ØņŚÉ ņØśĒĢśņŚ¼ ņŗØļÅä ņĀÉļ¦ē ņåÉņāüņØ┤ ļ░£ņāØĒĢ£ Ļ▓ĮņÜ░ ņĀÉļ¦ēĒĢś ņĪ░ņ¦üņØ┤ ņŗØļÅä ņäĖĻĘĀņ┤ØņŚÉ ņśüĒ¢źņØä ļ░øņØä ņłś ņ׳ļŗż[4]. ņŗØļÅä ņäĖĻĘĀņ┤ØņØĆ ļæÉ ĻĄ░ņ£╝ļĪ£ ļéśļłäņ¢┤ņ¦ł ņłś ņ׳ļŖöļŹ░ IĒśĢ ņäĖĻĘĀņ┤ØņØĆ ņŻ╝ļĪ£ StreptococcusļĪ£ ĻĄ¼ņä▒ļÉśļ®░ ņĀĢņāü ņŗØļÅäņŚÉņä£ Ļ┤Ćņ░░ļÉ£ļŗż. IIĒśĢ ņäĖĻĘĀņ┤ØņØĆ Veillonella, Prevotella, Haemophilus, Neisseria, Rothia, Granulicatella, Campylobacter, Porphyromonas, Fusobacterium, ActinomycesņÖĆ Ļ░ÖņØĆ ĻĘĖļ×ī ņØīņä▒ ĒśÉĻĖ░ņä▒ Ēś╣ņØĆ ļ»ĖĒśĖĻĖ░ņä▒(microaerophiles) ĻĘĀļōżļĪ£ ņŗØļÅäņŚ╝(odds ratio [OR], 15.4)ņØ┤ļéś ļ░öļĀøņŗØļÅä(OR, 16.5)ņÖĆ Ļ░ÖņØĆ ļ╣äņĀĢņāü ņŗØļÅäņÖĆ ņŚ░Ļ┤ĆļÉ£ļŗż[12]. ĒĢśļČĆ ņŗØļÅä ņäĖĻĘĀņ┤ØņØ┤ IĒśĢņŚÉņä£ IIĒśĢņ£╝ļĪ£ ņ¦äĒ¢ēĒĢśļŖö Ļ▓āņØĆ NF-kB Ļ▓ĮļĪ£ļź╝ ĒåĄĒĢśņŚ¼ ņŚ╝ņ”Ø ļ░śņØæņØ┤ ņ£Āļ░£ļÉśļ®┤ņä£ ņŗØļÅäņŚ╝ņŚÉņä£ ļ░öļĀøņŗØļÅäļź╝ Ļ▒░ņ│É ņŗØļÅäņäĀņĢöņØ┤ ļ░£ņāØĒĢśļŖö ņ¦äĒ¢ē Ļ│╝ņĀĢņŚÉ ņŚ░Ļ┤ĆļÉśļŖö Ļ▓āņ£╝ļĪ£ ņČöņĀĢļÉ£ļŗż[4]. ĻĄ¼Ļ░Ģ ņäĖĻĘĀņ┤ØĻ│╝ ņŗØļÅäņĢöņØś Ļ┤ĆĻ│äļź╝ ņĪ░ņé¼ĒĢ£ ņżæĻĄŁ ņŚ░ĻĄ¼ņŚÉņä£ ĻĄ¼Ļ░Ģ ļé┤ Tannerella forsythiaņØś ņ”ØĻ░ĆĻ░Ć ņŗØļÅäņäĀņĢö ņ£äĒŚśņä▒Ļ│╝ ņŚ░Ļ┤ĆļÉśņŚłĻ│Ā Porphyromonas gingivalis ņØś ņ”ØĻ░ĆļŖö ņŗØļÅäĒÄĖĒÅēņāüĒö╝ņäĖĒżņĢö ņ£äĒŚśņä▒Ļ│╝ ņŚ░Ļ┤ĆļÉśņŚłļŗż[13]. ņżæĻĄŁņØś ļŗżļźĖ ņŚ░ĻĄ¼ņŚÉņä£ļŖö ĻĄ¼Ļ░Ģ ļé┤ Lautropia, Bulleidia, Catonella, Corynebacterium, Moryella, Peptococcus ļ░Å CardiobacteriumņØś Ļ░ÉņåīĻ░Ć ņŗØļÅäĒÄĖĒÅēņāüĒö╝ņäĖĒżņĢö ļ░£ņāØĻ│╝ ņŚ░Ļ┤ĆļÉśņŚłļŗż[14]. ņØ┤ļ×Ć ņŚ░ĻĄ¼ņŚÉņä£ļŖö ņŗØļÅäņŚ╝ ĒÖśņ×Éļéś Ļ▒┤Ļ░ĢņØĖņŚÉ ļ╣äĒĢśņŚ¼ ņŗØļÅäĒÄĖĒÅēņāüĒö╝ņäĖĒżņĢö ĒÖśņ×ÉņØś ņ£ä ņäĖĻĘĀņ┤ØņŚÉ ClostridialesĻ│╝ ErysipelotrichalesĻ░Ć ļ¦ÄņĢśļŗż[15]. ļśÉĒĢ£ ņŗØļÅäĒÄĖĒÅēņāüĒö╝ ņĢö ņĪ░ņ¦üņŚÉņä£ Streptococcus ļ░Å PrevotellaĻ░Ć ņ”ØĻ░ĆĒĢśļŖö Ļ▓āņØĆ ļéśņü£ ņśłĒøä ņØĖņ×ÉņÖĆ ņŚ░Ļ┤ĆļÉśņŚłļŗż[16].
ņ£äļŖö ļé«ņØĆ ņé░ļÅäņÖĆ ļŗżņ¢æĒĢ£ ņåīĒÖö ĒÜ©ņåīĻ░Ć ņĪ┤ņ×¼ĒĢśļŖö ĒÖśĻ▓Į ļĢīļ¼ĖņŚÉ Ļ│╝Ļ▒░ņŚÉļŖö ĻĘĀņØ┤ ņāØņĪ┤ĒĢśĻĖ░ ņ¢┤ļĀżņÜ┤ Ļ││ņ£╝ļĪ£ ņŚ¼Ļ▓©ņĪīņ£╝ļéś H. pyloriĻ░Ć ļ░£Ļ▓¼ļÉśļ®┤ņä£ ĻĘĀņØ┤ ņĪ┤ņ×¼ĒĢĀ ņłś ņ׳ļŗżļŖö ņé¼ņŗżņØ┤ ļ░ØĒśĆņĪīļŗż. ĻĘĖļ¤¼ļéś ņĢäņ¦üĻ╣īņ¦Ć ļīĆļ│ĆņØä ņØ┤ņÜ®ĒĢ£ ļīĆņן ņäĖĻĘĀņ┤Ø ņŚ░ĻĄ¼ņŚÉ ļ╣äĒĢśņŚ¼ ņ£ä ņäĖĻĘĀņ┤ØņŚÉ ļīĆĒĢ£ ņŚ░ĻĄ¼ļōżņØĆ ļ¦Äņ¦Ć ņĢŖļŗż. ņ£ä ņäĖĻĘĀņ┤ØņØĆ ĻĄ¼Ļ░ĢņØ┤ļéś ņŗØļÅä ĻĘĖļ”¼Ļ│Ā ņåīņןĻ│╝ļŖö ļŗżļźĖ ņĪ░ņä▒ņØä Ļ░Ćņ¦ĆĻ│Ā ņ׳ļŗż. ņ£ä ņäĖĻĘĀņ┤ØņØś ņĪ░ņä▒ņŚÉļŖö H. pylori ņ£Āļ¼┤Ļ░Ć Ļ░Ćņן Ļ▓░ņĀĢņĀüņØĖ ņÜöņØĖņ£╝ļĪ£ ņ×æņÜ®ĒĢśļ®░, H. pyloriĻ░Ć ņŚåļŖö Ļ▓ĮņÜ░ņŚÉļŖö ļŗżņ¢æĒĢ£ ĻĘĀļōżņØ┤ ņ£ä ļé┤ņŚÉ ņĪ┤ņ×¼ĒĢ£ļŗż. ļČüļ»Ė ņøÉņŻ╝ļ»╝ņØä ļīĆņāüņ£╝ļĪ£ ĒĢ£ ņŚ░ĻĄ¼ņŚÉņä£ļŖö H. pyloriĻ░Ć ņØīņä▒ņØĖ Ļ▓ĮņÜ░ ActinobacteriaņÖĆ FirmicutesĻ░Ć ļŹö ņÜ░ņäĖĒĢśņśĆĻ│Ā, H. pyloriĻ░Ć ņ¢æņä▒ņØĖ Ļ▓ĮņÜ░ non-H. pylori ProteobacteriaņÖĆ AcidobacteriaĻ░Ć ņÜ░ņäĖĒĢśņśĆļŗż[17]. ĻĘĖļ¤¼ļéś H. pylori Ļ░ÉņŚ╝ ņ£Āļ¼┤Ļ░Ć ņ£ä ņäĖĻĘĀņ┤ØņŚÉ ņśüĒ¢źņØä ņŻ╝ņ¦Ć ņĢŖļŖöļŗżļŖö ņŚ░ĻĄ¼ļÅä ņ׳ļŗż[18]. H. pylori ņ£Āļ¼┤ņŚÉ Ļ┤ĆĻ│äņŚåņØ┤ Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes ļ░Å FusobacteriaĻ░Ć ņ£ä ļé┤ņŚÉ ņĪ┤ņ×¼ĒĢśļŖö ņŻ╝ņÜö ļ¼Ė(phylum)ņØ┤ļŗż[17,18]. ĒĢśņ£ä ņłśņżĆņŚÉņä£ļŖö Ļ▓Ćņé¼ ļ░®ļ▓ĢĻ│╝ ļīĆņāüņŚÉ ļö░ļØ╝ ļŗżņ¢æĒĢ£ ĻĘĀļōżņØś ņĪ┤ņ×¼ļź╝ ĒÖĢņØĖĒĢĀ ņłś ņ׳ļŖöļŹ░[19], ņśłļź╝ ļōżļ®┤ ņåīĒÖöļČłļ¤ēņ”Ø ĒÖśņ×Éļź╝ ļīĆņāüņ£╝ļĪ£ ĒĢ£ ņåŹ(genus) ņłśņżĆ ņŚ░ĻĄ¼ņŚÉņä£ļŖö Propionibacterium, Lactobacillus, Streptococcus ļ░Å StaphylococcusĻ░Ć ņŻ╝ļÉ£ ĻĘĀņŻ╝ņśĆļŗż[20]. ņ£äņĢö ļ░£ņāØņŚÉ H. pyloriĻ░Ć Ļ░Ćņן ņżæņÜöĒĢ£ ņÜöņØĖņØ┤ļØ╝ļŖö Ļ▓āņØĆ ņØśņŗ¼ĒĢĀ ļ░ö ņŚåņ£╝ļéś ļ¦īņä▒ ņ£äņŚ╝, ņןņāüĒö╝ĒÖöņāØ, ņ£äņĢöņŚÉņä£ Ļ░üĻ░ü ņ£ä ņäĖĻĘĀņ┤ØņØ┤ ņä£ļĪ£ ļŗżļź┤ļŗżļŖö Ļ▓āņØ┤ ĻĄŁļé┤ ļ░Å ņÖĖĻĄŁņØś ĒÖśņ×ÉņŚÉņä£ ļ│┤Ļ│ĀļÉśņŚłļŗż[21-23]. ĻĄŁļé┤ ĒÖśņ×ÉļōżņØä ļīĆņāüņ£╝ļĪ£ ĒĢ£ ņŚ░ĻĄ¼ņŚÉņä£ļŖö ņ£äņĢö ĒÖśņ×ÉņØś ņ£äņŚÉņä£ ņ£äņŚ╝ņØ┤ļéś ņןņāüĒö╝ĒÖöņāØ ĒÖśņ×ÉņŚÉ ļ╣äĒĢśņŚ¼ Bacillus Ļ░Ģ(class)Ļ│╝ Streptococcaceae Ļ│╝(family)Ļ░Ć ņ”ØĻ░ĆĒĢśņśĆļŗż[22]. Ēżļź┤Ēł¼Ļ░ł ņ£äņĢö ĒÖśņ×ÉņŚÉņä£ļŖö ņ£äņŚ╝ ĒÖśņ×ÉņŚÉ ļ╣äĒĢśņŚ¼ H. pyloriĻ░Ć Ļ░ÉņåīĒĢśļ®┤ņä£ ņāüļīĆņĀüņ£╝ļĪ£ Phyllobacterium, Achromobacter ņåŹņØ┤ļéś Xanthomonadaceae ļ░Å Enterobacteriaceae Ļ│╝Ļ░Ć ĒżĒĢ©ļÉ£ Proteobacteria ļ¼ĖņØ┤ ņ”ØĻ░ĆļÉśņŚłļŗż[21]. ļśÉ ņ£äņĢöņŚÉņä£ļŖö ņĀĢņāüņØ┤ļéś ņ£äņŚ╝ņŚÉ ļ╣äĒĢśņŚ¼ ņ£ä ņäĖĻĘĀņ┤Ø ļČłĻĘĀĒśĢņØ┤ ņŗ¼ĒĢśņśĆĻ│Ā[21-23] ļ®öĒāĆĻ▓īļåł ĻĖ░ļŖź ļČäņäØņŚÉņä£ļÅä ņ¦łņé░ņŚ╝ ĒÖśņøÉĒÜ©ņåī(nitrate reductase)ņÖĆ ņĢäņ¦łņé░ņŚ╝ ĒÖśņøÉĒÜ©ņåī(nitrite reductase) ĻĖ░ļŖźņØ┤ ņ”ØĻ░ĆļÉśņ¢┤ ņ׳ņŚłļŗż[21]. ņ£äņĢö ĒÖśņ×ÉņŚÉņä£ H. pylori ņØ┤ņÖĖņŚÉ ņ”ØĻ░ĆĒĢ£ ļŗżļźĖ ĻĘĀļōżņØ┤ ļ░£ņĢö Ļ│╝ņĀĢņŚÉ ņ¢┤ļ¢ż ņŚŁĒĢĀņØä ĒĢśļŖöņ¦Ć ņĢäņ¦ü ļ░ØĒśĆņ¦Ćņ¦Ć ņĢŖņĢśĻĖ░ ļĢīļ¼ĖņŚÉ Ē¢źĒøä ĻĖ░ņĀä ņŚ░ĻĄ¼Ļ░Ć ļŹö ņØ┤ļŻ©ņ¢┤ņĀĖņĢ╝ ĒĢ£ļŗż.
H. pyloriļŖö ņ£äņČĢņä▒ ņ£äņŚ╝, ņåīĒÖöņä▒ ĻČżņ¢æĻ│╝ Ļ░ÖņØĆ ņ¢æņä▒ ņ¦łĒÖś ļ░Å ņ£äņĢöņØ┤ļéś ļ│ĆņŚ░ļČĆ B ņäĖĒż ļ”╝Ēöäņóģ(mucosa-associated lymphoid tissue type)Ļ│╝ Ļ░ÖņØĆ ņĢģņä▒ ņ¦łĒÖśņØś ņøÉņØĖņØ┤ ļÉĀ ņłś ņ׳ļŗż. ĻĘĖļ¤¼ļ»ĆļĪ£ ņØ┤ņÖĆ ņŚ░Ļ┤ĆļÉ£ ņ¦łĒÖśļōżņØś ņ╣śļŻī ļ░Å ņśłļ░®ņØä ņ£äĒĢśņŚ¼ H. pylori ņĀ£ĻĘĀ ņ╣śļŻīĻ░Ć ļ¦żņÜ░ ņżæņÜöĒĢśļŗż. ĻĄŁļé┤ņŚÉņä£ļŖö ņ¢æņä▒ņ×ÉĒÄīĒöäņ¢ĄņĀ£ņĀ£, amoxicillin, clarithromycinņØä Ļ░ÖņØ┤ ņé¼ņÜ®ĒĢśļŖö ņé╝ņĀ£ņÜöļ▓ĢņØ┤ 1ņ░© ņ╣śļŻīļĪ£ ņé¼ņÜ®ļÉśĻ│Ā ņ׳ņ¦Ćļ¦ī ņĀ£ĻĘĀņ£©ņØ┤ ņĀÉņ░©ņĀüņ£╝ļĪ£ Ļ░ÉņåīĒĢśļŖö Ļ▓āņØ┤ ļ¼ĖņĀ£Ļ░Ć ļÉśĻ│Ā ņ׳ļŗż[24]. ņØ┤ļ¤¼ĒĢ£ ņĀ£ĻĘĀ ņŗżĒī©ņØś ņŻ╝ņÜö ņøÉņØĖņØĆ ĒĢŁņāØņĀ£ ļé┤ņä▒ņØ┤ĻĖ░ ļĢīļ¼ĖņŚÉ H. pylori ņĀ£ĻĘĀņ£©ņØä Ē¢źņāüņŗ£ĒéżĻĖ░ ņ£äĒĢśņŚ¼ Ļ░ĢļĀźĒĢ£ ņ£äņé░ ņ¢ĄņĀ£ņĀ£ņØś ņé¼ņÜ®, ņł£ņ░© ņ╣śļŻī, ļÅÖņŗ£ ņ╣śļŻī, ņāłļĪ£ņÜ┤ ĒĢŁņāØņĀ£ņØś ņé¼ņÜ® ļō▒ņØś ņĀäļץņØ┤ ņŗ£ļÅäļÉśĻ│Ā ņ׳ļŗż. ņØ┤ļ¤¼ĒĢ£ ļģĖļĀźņØś ņØ╝ĒÖśņ£╝ļĪ£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżļź╝ ņĀ£ĻĘĀ ņ╣śļŻīņÖĆ ĒĢ©Ļ╗ś ņé¼ņÜ®ĒĢ┤ ņÖöņ£╝ļ®░, ĻĘĖ ĻĖ░ņĀäņØĆ H. pyloriņŚÉ ļīĆĒĢ£ ņ¦üņĀæ ņ¢ĄņĀ£ ĒÜ©Ļ│╝, ņĀÉļ¦ē ļ░®ņ¢┤ Ļ░ĢĒÖö ĒÜ©Ļ│╝, ļ®┤ņŚŁ ļ░śņØæ ņĪ░ņĀł ĒÜ©Ļ│╝, IgA ļČäļ╣ä ļō▒Ļ│╝ ĒĢ©Ļ╗ś ĒĢŁņāØņĀ£ ņé¼ņÜ®Ļ│╝ ņŚ░Ļ┤ĆļÉ£ ļČĆņ×æņÜ®ņØä Ļ░Éņåīņŗ£ņ╝£ ņĢĮņĀ£ ļ│ĄņÜ® ņł£ņØæļÅäļź╝ ļåÆņØ┤ļŖö Ļ▓ā ļō▒ņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż[25,26]. ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖż ļŗ©ļÅģ Ēł¼ņŚ¼ļŖö H. pylori ĒÖ£ņä▒ņØä ļé«ņČ£ ņłśļŖö ņ׳ņ£╝ļéś ņĀ£ĻĘĀņØä ņŗ£Ēéżņ¦ĆļŖö ļ¬╗ĒĢśĻĖ░ ļĢīļ¼ĖņŚÉ Ēæ£ņżĆ ņé╝ņĀ£ņÜöļ▓ĢņØä ĻĖ░ļ│Ėņ£╝ļĪ£ ĒĢśĻ│Ā ņČöĻ░ĆļĪ£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżļź╝ Ēł¼ņŚ¼ĒĢśĻ▓ī ļÉśļŖöļŹ░, ņØ┤ļ¤░ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖż ņČöĻ░Ć Ēł¼ņŚ¼Ļ░Ć ņĀ£ĻĘĀņ£©ņØä Ē¢źņāüņŗ£ĒéżļŖöņ¦Ć ĻŠĖņżĆĒ׳ ņŚ░ĻĄ¼ļÉśĻ│Ā ņ׳ļŗż[27]. ņĄ£ĻĘ╝ 3ļģä ļÅÖņĢł ļ░£Ēæ£ļÉ£ 7Ļ░£ņØś ļ®öĒāĆļČäņäØ ļ¬©ļæÉ ņĀ£ĻĘĀ ņ╣śļŻīņÜöļ▓ĢņŚÉ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżļź╝ ņČöĻ░ĆĒĢśļŖö Ļ▓ĮņÜ░ ņĀ£ĻĘĀņ£©ņØä Ļ░£ņäĀņŗ£ņ╝░ļŗżĻ│Ā ļ│┤Ļ│ĀĒĢśņśĆļŗż(relative risk [RR], 1.12~1.85) (Table 1) [25,28-38]. ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖż ņČöĻ░Ć Ēł¼ņŚ¼ņØś ņżæņÜöĒĢ£ ĒÜ©Ļ│╝ļŖö ĒĢŁņāØņĀ£ņÖĆ ņŚ░Ļ┤ĆļÉ£ ļČĆņ×æņÜ®ņØä Ļ░Éņåī(RR, 0.40~0.45)ņŗ£ņ╝£ ņĢĮņĀ£ ļ│ĄņÜ® ņł£ņØæļÅäļź╝ ļåÆņØ╝ ņłś ņ׳ļŗżļŖö Ļ▓āņØĖļŹ░, ĒŖ╣Ē׳ ņäżņé¼ ņ”ØņāüņØĆ ļīĆļČĆļČäņØś ņŚ░ĻĄ¼ņŚÉņä£ Ļ░ÉņåīļÉśņŚłļŗż(RR, 0.3~0.54). ĒĢ£ ļ®öĒāĆļČäņäØņŚÉņä£ļŖö Ēæ£ņżĆ ņé╝ņĀ£ņÜöļ▓ĢņŚÉ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖż ļ│ĄĒĢ® ĻĘĀņŻ╝ļź╝ Ļ░ÖņØ┤ ņé¼ņÜ®ĒĢ£ Ļ▓ĮņÜ░ H. pylori ņĀ£ĻĘĀ ņä▒Ļ│ĄņŚÉ ļīĆĒĢ£ number needed to treatļŖö 10.2ņśĆĻ│Ā, ĒĢŁņāØņĀ£ ņŚ░Ļ┤Ć ļČĆņ×æņÜ®ņØä ņĢĮ 14% ņĀĢļÅä Ļ░Éņåīņŗ£ņ╝░ļŗż[32]. Ēśäņ×¼Ļ╣īņ¦ĆņØś ņŚ░ĻĄ¼ļōżņØä ņóģĒĢ®ĒĢ┤ļ│┤ļ®┤ ļŗżņ¢æĒĢ£ ņóģļźśņØś ļŗ©ņØ╝ Ēś╣ņØĆ ļ│ĄĒĢ® ĻĘĀņŻ╝ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżĻ░Ć ļÅÖņ¢æ ļ░Å ņä£ņ¢æņØś ņŚ░ĻĄ¼ ļ¬©ļæÉņŚÉņä£ ņĀ£ĻĘĀņ£©ņØä ņ”ØĻ░Ćņŗ£ĒéżĻ│Ā ĒĢŁņāØņĀ£ ņŚ░Ļ┤Ć ņäżņé¼ļź╝ Ļ░Éņåīņŗ£ņ╝░ņ¦Ćļ¦ī ĻĘĖ ĒÜ©Ļ│╝ļŖö Ēü¼ņ¦Ć ņĢŖņĢśļŗż[25].
ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”ØņØś ļ░£ņāØ ĻĖ░ņĀäņ£╝ļĪ£ ļé┤ņן Ļ│╝ļ»╝ņä▒, ņ£äļ░░ņČ£ ņןņĢĀ, ņ£äņĪ░ņĀł ņןņĢĀ ļō▒ņØ┤ ņĢīļĀżņĀĖ ņ׳ļŗż. ņĄ£ĻĘ╝ ĻĖ░ļŖźņä▒ ņ£äņןĻ┤Ć ņ¦łĒÖśņŚÉņä£ ņןļé┤ ņäĖĻĘĀņØś ņŚŁĒĢĀņØ┤ Ļ░ĢņĪ░ļÉśļ®┤ņä£ ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”Ø ĒÖśņ×ÉņØś ņ£ä ņäĖĻĘĀņ┤Ø ļ│ĆĒÖöļź╝ Ļ┤Ćņ░░ĒĢ£ ņåīĻĘ£ļ¬© ņŚ░ĻĄ¼ļōżņØ┤ ņŗ£Ē¢ēļÉśņŚłļŗż. ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”Ø ĒÖśņ×Éļź╝ ļīĆņāüņ£╝ļĪ£ terminal restriction fragment length polymorphismņØä ņØ┤ņÜ®ĒĢ£ ņŚ░ĻĄ¼ņŚÉņä£ Bifidobacterium ņ”ØĻ░Ć, Prevotella Ļ░Éņåī, Clostridium IX, XI, XVIII ņ”ØĻ░ĆņÖĆ Ļ░ÖņØĆ ņ£ä ņäĖĻĘĀņ┤Ø ņØ┤ņāüņØ┤ Ļ┤Ćņ░░ļÉśņŚłĻ│Ā[3], ņ░©ņäĖļīĆ ņŚ╝ĻĖ░ ļČäņäØ ļ░®ļ▓ĢņØä ņØ┤ņÜ®ĒĢ£ ņŚ░ĻĄ¼ņŚÉņä£ļŖö Bacteriodetes ņ”ØĻ░Ć, Proteobacteria Ļ░Éņåī, Acidobacteria Ļ░ÉņåīĻ░Ć Ļ┤Ćņ░░ļÉśņŚłļŗż. ņåŹ ņłśņżĆņŚÉņä£ļŖö Bacteroides ņ”ØĻ░ĆņÖĆ Edaphobacter Ļ░ÉņåīĻ░Ć Ļ┤Ćņ░░ļÉśņŚłĻ│Ā ņóģ(species) ņłśņżĆņŚÉņä£ļŖö Bacteriodes dorei ļ░Å Escherichia coli/shigella ņ”ØĻ░Ć ĻĘĖļ”¼Ļ│Ā Edaphobacter aggregans Ļ░ÉņåīĻ░Ć Ļ┤Ćņ░░ļÉśņŚłļŗż. ĒØźļ»ĖļĪŁĻ▓īļÅä ņØ┤ļ¤¼ĒĢ£ ņ£ä ņäĖĻĘĀņ┤ØņØś ņØ┤ņāüņØĆ Lactobacillus gasseri OLL2716 ņÜöĻ▒░ĒŖĖļź╝ Ēł¼ņŚ¼ĒĢśņśĆņØä ļĢī ņĀĢņāüņØĖĻ│╝ ļ╣äņŖĘĒĢśĻ▓ī ĒÜīļ│ĄļÉśņŚłļŗż[39]. ņĄ£ĻĘ╝ ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ē ņ”ØņāüņØä ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżĻ░Ć ĒśĖņĀäņŗ£Ēé¼ ņłś ņ׳ļŖöņ¦ĆņŚÉ ļīĆĒĢśņŚ¼ ņåīĻĘ£ļ¬© ņŚ░ĻĄ¼Ļ░Ć ļ░£Ēæ£ļÉśņŚłļŗż. H. pylori Ļ░ÉņŚ╝ņØ┤ ņŚåļŖö ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”Ø ĒÖśņ×ÉņŚÉĻ▓ī Lactobacillus gasseri OLL2716 ņÜöĻ▒░ĒŖĖļź╝ Ēł¼ņŚ¼ĒĢśļ®┤ 12ņŻ╝ņ¦ĖņŚÉ ņ£ĀņØśĒĢśĻ▓ī ņ£äņĢĮļ│┤ļŗż ņ”Øņāü ĒśĖņĀäņ£©ņØ┤ ļåÆņĢśļŗż. ņŗØĒøä ĒīĮļ¦īĻ░É, ņĪ░ĻĖ░ Ēżļ¦īĻ░É ļō▒ ņŗØĒøä ļČłĒÄĖ ņ”ØĒøäĻĄ░(postprandial distress syndrome) ņ”ØņāüņØä ĒśĖņĀäņŗ£ņ╝░ņ£╝ļéś ņāüļ│ĄļČĆ ĒåĄņ”ØĻ│╝ ņåŹņō░ļ”╝ņØĆ ņ░©ņØ┤Ļ░Ć ņŚåņŚłļŗż[40,41]. Bifidobacterium bifidum YIT 10347 ļ░£ĒÜ©ņ£Āļź╝ 4ņŻ╝Ļ░ä ļ│ĄņÜ®ĒĢ£ ļæÉ ņŚ░ĻĄ¼ņŚÉņä£ļŖö ņåīĒÖöļČłļ¤ē, ņ£äņé░Ļ│╝ ņŚ░Ļ┤ĆļÉ£ ņ”Øņāü, ņŗØĒøä ļČłĒÄĖĻ░ÉĻ│╝ ņāüļ│ĄļČĆ ĒåĄņ”ØņØ┤ ĒśĖņĀäļÉśņŚłņØä ļ┐Éļ¦ī ņĢäļŗłļØ╝ ņĀĢņŗĀņĀüņØĖ ņ”ØņāüļÅä Ļ░ÖņØ┤ ĒśĖņĀäļÉśņŚłļŗż[42,43]. ņżæļ»Ė ņ¦ĆņŚŁņŚÉņä£ ņłśĒ¢ēļÉ£ ņŚ░ĻĄ¼ņŚÉņä£ Bacillus coagulans ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżļź╝ ĒÖśņ×ÉņŚÉĻ▓ī Ēł¼ņŚ¼ĒĢśļ®┤ ņ£äņןĻ┤Ć ņ”Øņāü ņĀÉņłśņØś ņ┤ØņĀÉĻ│╝ ļ│ĄĒåĄ ņĀÉņłśĻ░Ć ĒśĖņĀäļÉśņŚłļŗż[44]. ĻĘĖļ¤¼ļéś Bifidobacterium bifidum YIT 10347ņÖĆ Bacillus coagulans ņŚ░ĻĄ¼ļŖö ļīĆņāü ĒÖśņ×ÉĻĄ░ņØ┤ Rome ĻĖ░ņżĆņØä ļ¦īņĪ▒ĒĢśņ¦Ć ļ¬╗ĒĢśĻ│Ā ņåīĒÖöļČłļ¤ē ņ”ØņāüņØä ņØ╝ņŗ£ņĀüņ£╝ļĪ£ Ļ░Ćņ¦ä ĒÖśņ×ÉļōżņØ┤ņŚłļŗżļŖö ņĀ£ĒĢ£ņĀÉņØ┤ ņ׳ņŚłļŗż. ņØ┤Ēāłļ”¼ņĢäņØś ņŚ░ĻĄ¼ņŚÉņä£ļŖö ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”Ø ĒÖśņ×ÉņŚÉĻ▓ī ņś¼ļ”¼ļĖīņ£ĀņÖĆ ĒĢ©Ļ╗ś Lactobacillus reuteri, Lactobacillus rhamnosus GG ļ░Å Saccharomyces boulardiiĻ░Ć ĒżĒĢ©ļÉ£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżļź╝ 7ņØ╝Ļ░ä Ēł¼ņŚ¼ĒĢ£ Ļ▓ĮņÜ░ ņś¼ļ”¼ļĖīņ£Ā ļŗ©ļÅģ Ēł¼ņŚ¼ļéś ņś¼ļ”¼ļĖīņ£ĀņÖĆ ĒĢŁņé░ĒÖöņĀ£ļź╝ Ļ░ÖņØ┤ Ēł¼ņŚ¼ĒĢ£ Ļ▓ĮņÜ░ņŚÉ ļ╣äĒĢśņŚ¼ ņ£ĀņØśĒĢśĻ▓ī ņśżņŗ¼, ņāüļ│ĄļČĆ ĒåĄņ”Ø, ņŗØĒøä ĒīĮļ¦ī, ĒŖĖļ”╝ ļō▒ņØś ņ”ØņāüņØ┤ ĒśĖņĀäļÉśņŚłļŗż[45].
ļīĆņןņØ┤ļéś ņ£äņŚÉ ļ╣äĒĢśņŚ¼ ņåīņןņØĆ ņĀæĻĘ╝ņØ┤ ļ¦żņÜ░ ņ¢┤ļĀĄĻĖ░ ļĢīļ¼ĖņŚÉ ņØĖĻ░äņØä ļīĆņāüņ£╝ļĪ£ ĒĢ£ ņåīņן ņäĖĻĘĀņ┤Ø ņŚ░ĻĄ¼ļŖö ĒÜīņןņĪ░ļŻ©ņłĀ ĒÖśņ×ÉņŚÉņä£ ņŗ£Ē¢ēļÉ£ Ļ▓ā ņÖĖņŚÉ Ļ▒░ņØś ņŚåļŗż. ļ╣äĻĄÉņĀü ņĢłņĀĢņĀüņØĖ ļīĆņן ņäĖĻĘĀņ┤ØņŚÉ ļ╣äĒĢśņŚ¼ ņåīņן ņäĖĻĘĀņ┤ØņØĆ ņØīņŗØ ņäŁņĘ©ņŚÉ ļö░ļØ╝ ņ¦¦ņØĆ ņŗ£Ļ░ä ļé┤ņŚÉ ņĪ░ņä▒ņØ┤ ļ░öļĆö ņłś ņ׳ļŗż. ņØĖĻ░ä ļīĆņāü ņŚ░ĻĄ¼ņŚÉņä£ļŖö ņåīņןņŚÉņä£ StreptococcusņÖĆ VeillonellaĻ░Ć ļ╣äĻĄÉņĀü ņØ╝Ļ┤ĆļÉśĻ▓ī Ļ┤Ćņ░░ļÉśļ®░ Clostridium, Escherichia ļ░Å TuricibacterļŖö ļŗżņ¢æĒĢ£ ļ╣łļÅäļĪ£ ņĪ┤ņ×¼ĒĢ£ļŗż[46]. ņ¦üņĀæ ņןņØä ņĀüņČ£ĒĢśņŚ¼ ņäĖĻĘĀņ┤ØņØä ņĪ░ņé¼ĒĢĀ ņłś ņ׳ļŖö ņāØņźÉ(mice) ņŚ░ĻĄ¼ņŚÉņä£ļŖö ņåīņןņŚÉ ņŻ╝ļĪ£ LactobacillaceaeĻ░Ć ņĪ┤ņ×¼ĒĢśļŖö Ļ▓āņØ┤ ĒÖĢņØĖļÉśņŚłļŗż[47]. ņåīņן ļé┤ ņäĖĻĘĀņ┤ØņØĆ ņØīņŗØņ£╝ļĪ£ ņäŁņĘ©ļÉ£ ļŗ©ļŗ╣ļźśļź╝ ļīĆņé¼ņŗ£ņ╝£ ļŗ©ņćäņ¦Ćļ░®ņé░ņØä ņāØņé░ĒĢśĻ│Ā ļŗżņ¢æĒĢ£ ļ®┤ņŚŁ ļ░śņØæņØä ņØ╝ņ£╝ĒéżļŖö ņŚŁĒĢĀņØä ĒĢśļŖö Ļ▓āņ£╝ļĪ£ ļ│┤ņØĖļŗż[46].
NSAIDļŖö H. pyloriņÖĆ ĒĢ©Ļ╗ś ņåīĒÖöņä▒ ĻČżņ¢æņØś Ļ░Ćņן ņżæņÜöĒĢ£ ņøÉņØĖ ņżæ ĒĢśļéśņØĖļŹ░, ņ£äņÖĆ ņŗŁņØ┤ņ¦Ćņן ņÖĖņŚÉļÅä ņåīņן ņĀÉļ¦ē ņåÉņāüņØä ņØ╝ņ£╝ņ╝£ ļ│ĄĒåĄņØ┤ļéś ņČ£ĒśłņØś ņøÉņØĖņØ┤ ļÉśĻĖ░ļÅä ĒĢ£ļŗż. NSAID Ēł¼ņŚ¼ļĪ£ ņåīņן ņĀÉļ¦ē ņåÉņāüņØ┤ ņØ╝ņ¢┤ļéśļŖö Ļ│╝ņĀĢņŚÉ ņןļé┤ ņäĖĻĘĀņØ┤ ņŚ░Ļ┤ĆļÉ£ļŗżļŖö Ļ▓āņØĆ ņśżļל ņĀäļČĆĒä░ ņĢīļĀżņĪīļŗż. ņĢĮ 40ļģä ņĀäņŚÉ ņŗ£Ē¢ēļÉ£ ņŚ░ĻĄ¼ņŚÉņä£ ņĀĢņāü ņןļé┤ ņäĖĻĘĀņ┤ØņØä Ļ░Ćņ¦ä ņźÉņŚÉ ļ╣äĒĢśņŚ¼ ļ¼┤ĻĘĀņźÉņŚÉņä£ļŖö indomethacin Ēł¼ņŚ¼ ĒøäņŚÉļÅä ņåīņן ņĀÉļ¦ē ņåÉņāüņØ┤ Ļ▓Įļ»ĖĒ¢łņ£╝ļ®░, ĒŖ╣Ē׳ ņłśņ╗Ę ļ¼┤ĻĘĀņźÉņŚÉņä£ļŖö ņĀÉļ¦ē ņåÉņāüņØ┤ ļ░£ņāØĒĢśņ¦Ć ņĢŖņĢśļŗż[48]. ĒøäņåŹ ņŚ░ĻĄ¼ņŚÉņä£ ļ¼┤ĻĘĀņźÉņŚÉ Escherichia coliļéś EubacteriumņØä ņØ┤ņŗØĒĢśĻ│Ā NSAIDļź╝ Ēł¼ņŚ¼ĒĢśļ®┤ ņåīņן ņĀÉļ¦ē ņåÉņāüņØ┤ ļ░£ņāØĒĢśņ¦Ćļ¦ī ĒĢŁņāØņĀ£ļź╝ Ļ░ÖņØ┤ Ēł¼ņŚ¼ĒĢśļ®┤ ļ░£ņāØĒĢśņ¦Ć ņĢŖņĢśĻ│Ā, BifidobacteriumņØ┤ļéś Lactobacillusļź╝ ņØ┤ņŗØĒĢśļŖö Ļ▓ĮņÜ░ņŚÉļÅä NSAID ņ£Āļ░£ ņåīņן ņĀÉļ¦ē ņåÉņāüņØ┤ ļ░£ņāØĒĢśņ¦Ć ņĢŖņĢśļŗż[49]. ņØ┤ļĀćĻ▓ī NSAID ņ£Āļ░£ ņåīņן ņĀÉļ¦ē ņåÉņāü ļ░£ņāØ Ļ│╝ņĀĢņŚÉ ĒŖ╣ņĀĢ ņןļé┤ ņäĖĻĘĀņØ┤ ņŚ░Ļ┤ĆļÉ£ļŗżļŖö Ļ▓░Ļ│╝ļŖö ņåīņן ņĀÉļ¦ē ņåÉņāüņØś Ļ▓ĮņÜ░ ņ£ä ņĀÉļ¦ē ņåÉņāüĻ│╝ļŖö ļŗżļźĖ ĻĖ░ņĀäņØ┤ ņ×æņÜ®ĒĢ£ļŗżļŖö Ļ▓āņØä ņŗ£ņé¼ĒĢ£ļŗż. ņ”ē NSAIDĻ░Ć prostaglandinņØä Ļ░Éņåīņŗ£ņ╝£ ņåīņן ņĀÉļ¦ēņØś ļ░®ņ¢┤ ĻĖ░ļŖźĻ│╝ Ēł¼Ļ│╝ļÅäņŚÉ ņØ┤ņāüņØ┤ ņāØĻĖ░ļ®┤ ņןļé┤ ņäĖĻĘĀņØ┤ļéś lipopolysaccharideņÖĆ Ļ░ÖņØĆ ņŚ╝ņ”Ø ņ£Āļ░£ ļ¼╝ņ¦łņØś ņĀÉļ¦ē ņ╣©Ēł¼Ļ░Ć ļ░£ņāØĒĢśĻ│Ā, ņØ┤ļĪ£ ņØĖĒĢśņŚ¼ ņĀÉļ¦ēĒĢśņŚÉņä£ tumor necrosis factor-╬▒, interleukin-1╬▓ ņ”ØĻ░ĆņÖĆ Ļ░ÖņØĆ ņŚ╝ņ”Ø ļ░śņØæņØ┤ ņØ╝ņ¢┤ļéś ņåīņן ņĀÉļ¦ēņäĖĒżļź╝ ņåÉņāüņŗ£ĒéżļŖö Ļ▓āņ£╝ļĪ£ ņČöņĀĢļÉśĻ│Ā ņ׳ļŗż[5]. Toll-like receptor 4ļéś myeloid differentiation primary-response 88 Ļ▓░ņåÉ ņāØņźÉ(knock out mice)ņŚÉņä£ļŖö NSAIDņŚÉ ņØśĒĢ£ ņåīņן ņĀÉļ¦ē ņåÉņāüņØ┤ ņĢĮĒĢśĻ▓ī ļéśĒāĆļéśĻĖ░ ļĢīļ¼ĖņŚÉ NSAID ņ£Āļ░£ ņåīņן ņĀÉļ¦ē ņåÉņāü Ļ│╝ņĀĢņØĆ toll-like receptor 4ņŚÉ ņØśņĪ┤ņĀüņØĖ Ļ▓āņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż[50]. NSAID ņ£Āļ░£ ņåīņן ņĀÉļ¦ē ņåÉņāüņŚÉ ļīĆĒĢ£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżņØś ĒÜ©Ļ│╝ļź╝ ĒÖĢņØĖĒĢ£ ņŚ░ĻĄ¼ļŖö ļ¦żņÜ░ ņĀüņØĆļŹ░, Lactobacillus casei ĻĘĀņŻ╝ļŖö ļÅÖļ¼╝ ņŗżĒŚśņŚÉņä£ ņĀÉļ¦ē ņåÉņāüņØä ņżäņśĆņØäļ┐Éļ¦ī ņĢäļŗłļØ╝ ņØĖĻ░ä ļīĆņāü ņŚ░ĻĄ¼ņŚÉņä£ļÅä ņåīņן ņ║ĪņŖÉļé┤ņŗ£Ļ▓ĮņŚÉņä£ ņĀÉļ¦ē ņåÉņāü ņĀÉņłśļź╝ ļé«ņČöņŚłļŗż[51,52]. ļśÉĒĢ£ ļ│ĄĒĢ® ĻĘĀņŻ╝ņØĖ VSL#3 ļ│ĄņÜ®ņØĆ ļ│ĄņÜ®ĒĢśņ¦Ć ņĢŖņØĆ ļīĆņĪ░ĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ NSAID Ēł¼ņŚ¼ Ēøä ļīĆļ│Ć calprotectin ņ¢æņØä ļŹö ļé«ņČöņŚłļŗż[53].
ņĢäņ¦üĻ╣īņ¦Ć ļīĆņן ņ¦łĒÖśņØ┤ļéś ļīĆņé¼ ņ¦łĒÖśņŚÉ ļ╣äĒĢśņŚ¼ ņāüļČĆņ£äņןĻ┤Ć ņ¦łĒÖśņŚÉņä£ļŖö ņןļé┤ ņäĖĻĘĀņ┤ØņØś ņŚŁĒĢĀņØ┤ ņל ļ░ØĒśĆņ¦Ćņ¦Ć ņĢŖņĢśļŗż. ļ░öļĀøņŗØļÅä, ņŗØļÅäņäĀņĢö, ņ£äņĢöĻ│╝ ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”ØņŚÉņä£ļŖö ņØ╝ļČĆ ņŚŁĒĢĀņØä ĒĢśļŖö Ļ▓āņ£╝ļĪ£ ļ│┤ņØ┤Ļ│Ā NSAIDņŚÉ ņØśĒĢ£ ņåīņן ņĀÉļ¦ē ņåÉņāü ļ░£ņāØ ĻĖ░ņĀäņŚÉļŖö ņżæņČöņĀüņØĖ ņŚŁĒĢĀņØä ĒĢ£ļŗż. ņāüļČĆņ£äņןĻ┤Ć ņ¦łĒÖśņØś ņ╣śļŻīņĀüņØĖ ņĖĪļ®┤ņŚÉņä£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖż ņé¼ņÜ®ņØĆ ņĢäņ¦ü ĻĘĖ ĒÜ©Ļ│╝Ļ░Ć ļČłļČäļ¬ģĒĢśļŗż. Ļ░Ćņן ĒØöĒ׳ ņé¼ņÜ®ļÉśļŖö Ļ▓ĮņÜ░ļŖö H. pylori ņĀ£ĻĘĀ ņÜöļ▓ĢņŚÉ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżļź╝ ņČöĻ░ĆĒĢśļŖö Ļ▓āņ£╝ļĪ£, ņäżņé¼ņÖĆ Ļ░ÖņØĆ ĒĢŁņāØņĀ£ ņŚ░Ļ┤Ć ļČĆņ×æņÜ®ņØä Ļ░Éņåīņŗ£ņ╝£ ļ│ĄņĢĮ ņł£ņØæļÅäļź╝ ļåÆņØ╝ ņłś ņ׳ņØä Ļ▓āņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż. ĻĖ░ļŖźņä▒ ņåīĒÖöļČłļ¤ēņ”ØņØ┤ļéś NSAIDņŚÉ ņØśĒĢ£ ņåīņן ņĀÉļ¦ē ņåÉņāüņŚÉņä£ļÅä ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżĻ░Ć ņ£ĀņÜ®ĒĢĀ ņłś ņ׳ļŗżļŖö ņ”ØĻ▒░ļōżņØ┤ ņĀ£ņŗ£ļÉśņŚłņ£╝ļéś ņĢäņ¦ü ņŚ░ĻĄ¼Ļ░Ć ļČĆņĪ▒ĒĢ£ ĒÄĖņØ┤ļŗż. ļśÉĒĢ£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżņØś ĒÜ©Ļ│╝ļŖö ĻĘĀņŻ╝ ĒŖ╣ņØ┤ņĀüņØ┤ĻĖ░ ļĢīļ¼ĖņŚÉ ĒŖ╣ņĀĢ ĻĘĀņŻ╝ņŚÉ ļīĆĒĢ£ ņŚ░ĻĄ¼ Ļ▓░Ļ│╝ļź╝ ļ¬©ļōĀ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżņŚÉ ļÅÖņØ╝ĒĢśĻ▓ī ņĀüņÜ®ĒĢĀ ņłśļŖö ņŚåļŗż. Ē¢źĒøä ņāüļČĆņ£äņןĻ┤Ć ņ¦łĒÖśņŚÉņä£ ņäĖĻĘĀņ┤ØņØś ņŚŁĒĢĀņØ┤ ļŹö ļ░ØĒśĆņ¦ĆĻ│Ā strain ņłśņżĆņŚÉņä£ ĒöäļĪ£ļ░öņØ┤ņśżĒŗ▒ņŖżņØś ņ×æņÜ® ĻĖ░ņĀäņØ┤ ļ░ØĒśĆņ¦Ćļ®┤ ņāüļČĆņ£äņןĻ┤Ć ņ¦łĒÖśņØś ņןļé┤ ņäĖĻĘĀ ĻĖ░ļ░ś ņ╣śļŻī ļ░®ļ▓Ģņ£╝ļĪ£ ņ£ĀņÜ®ĒĢśĻ▓ī ņé¼ņÜ®ļÉĀ ņłś ņ׳ņØä Ļ▓āņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż.
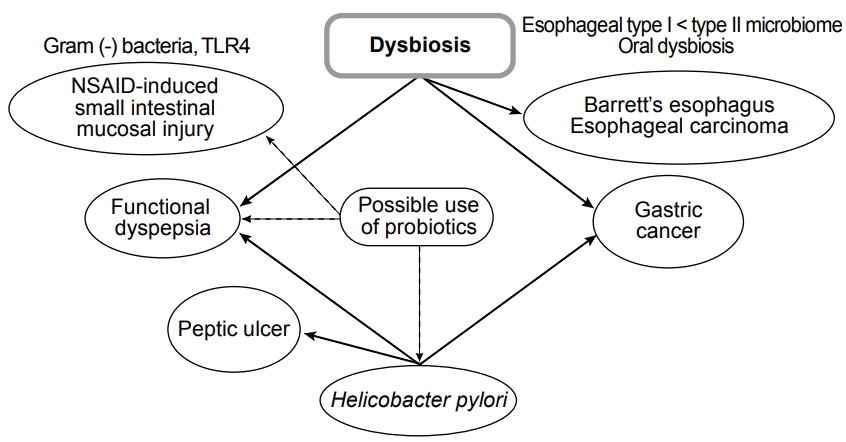
Fig.┬Ā1.
Upper gastrointestinal tract disease and dysbiosis. TLR, toll-like receptor; NSAID, nonsteroidal anti-inflammatory drug.
Table┬Ā1.
Summary of the Meta-analyses Published from 2016 on the Effect of Probiotics in Helicobacter pylori Eradication Therapy
| Author | Year | Number of included studies | Comparison | Probiotics straina | RR (95% CI) for eradication rate | RR (95% CI) for side effects |
|---|---|---|---|---|---|---|
| Fang et al. [28] | 2019 | 5 | Triple therapy with Lactobacillus vs. triple therapy with placebo or not | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus Thamnosus, Lactobacillus GG, Lactobacillus reuteri | 1.19 (1.07, 1.33) | Diarrhea 0.30 (0.10, 0.85) |
| Wen et al. [29] | 2017 | 17 | Triple therapy with probiotics vs. triple therapy with placebo | L. acidophilus, Lactobacillus bulgaricus, L. casei, Lactobacillus delbrueckii, L. reuteri, Bifidobacterium animalis, Bifidobacterium bifidum, Bifidobacterium inf antis, Bifidobacterium longum, Lactococcus lactis, Bacillus mesentericus, Clostridium butyricum, Enterococcus faecalis, Streptococcus faecalis, Streptococcus thermophilus, Saccharomyces boulardii | 1.16 (1.07, 1.26) | Overall 0.40 (0.34, 0.48) |
| Feng et al. [30] | 2017 | 29 | Triple therapy with probiotics vs. triple dierapy with placebo | L. acidophilus, L. bulgaricus, L. casei, Lactobacillus plantarum, L. rhamnosus, L. reuteri, Lactobacillus salivarius, Lactobacillus sporogenes, B. bifidum, B. infantis, B. longum, B. mesentericus, C. butyricum, S. faecalis, S. thermophilus, S. boulardii | ITT 1.19 (1.13, 1.25) | Overall 0.49 (0.38, 0.65) |
| PP 1.19 (1.13, 1.25) | ||||||
| Lau et al. [31] | 2016 | 30 | Triple therapy with probiotics vs. triple therapy with placebo | L. acidophilus, L. casei, L. reuteri, L. rhamnosus GG, Lactobacillus paracasei, L. plantarum, L. salivarius, L. sporogenes, B. animalis, B. bifidum, Bifidobacterium breve, B. lactis, B. longum, Bacillus clausii, Streptococcus faecium, S. thermophiles, Propionibacterium freudenreichii, S. boulardii, Kefir | ITT 1.14 (1.11, 1.18) | Nausea 0.61 (0.52, 0.71) |
| PP 1.12 (1.09, 1.15) | Vomiting 0.72 (0.53, 0.99) | |||||
| Diarrhea 0.55 (0.39, 0.77) | ||||||
| Epigastric pain 0.81 (0.72, 0.91) | ||||||
| L├╝ et al. [25] | 2016 | 13 | Any regimen with probiotics vs. any regimen with placebo | L. acidophilus, L. acidophilus HY2177, L. acidophilus La5, L. bulgaricus, L. casei, L. casei HY2743, Lactobacillus gasseri OLL2716, L. Thamnosus, L. reuteri, L. reuteri ATCC55730, B. lactis Bb12, B. breve, B. bifidum, B. infantis, B. longum, B. longum HY8001, S. faecium, S. thermophiles | ITT 1.15 (1.10, 1.20) | Overall 0.71 (0.54, 0.94) |
| Nausea & vomiting 0.58 (0.35, 0.95) | ||||||
| Diarrhea 0.51 (0.31, 0.84) | ||||||
| Constipation 0.47 (0.28, 0.80) | ||||||
| McFarland et al. [32] | 2016 | 19 | Any regimen with multi-strain probiotics vs. any regimen with placebo or not | L. acidophilus, L. acidophilus HY2177, L. acidophilus La5, L. casei HY2743, Lactobacillus casei rhamnosus, Lactobacillus helveticus R0052, L. plantarum, L. reuteri, L. rhamnosus R011, L. salivarius, L. sporogenes, B. animalis subsp. lactis Bb12, B. bifidum, B. infantis, B. longum HY8001, E. Faecalis | ITT 1.12 (1.08, 1.17) | Overall 0.45 (0.30, 0.65) |
| Lu et al. [33] | 2016 | 21 | Triple therapy with probiotics vs. triple therapy with placebo | L. acidophilus, L. GG, L. rhamnosus, B. bifidum, S. boulardii, B. breve, B. clausii, S. faecium, P. freudenreichii | Not statistically significant | Nausea 0.36 (0.21, 0.62) |
| Diarrhea 0.33 (0.19, 0.57) | ||||||
| Bloating 0.5 (0.3, 0.83) | ||||||
| Triple therpy with probiotics vs. triple therapy alone | ITT 1.84 (1.51, 2.25) | Nausea 0.43 (0.27, 0.7) | ||||
| PP 1.85 (1.47, 2.31) | Vomiting 0.3 (0.11, 0.86) | |||||
| Diarrhea 0.43 (0.21, 0.89) | ||||||
| Constipation 0.28 (0.13, 0.64) |
REFERENCES
1. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369ŌĆō2379.


2. Coker OO, Dai Z, Nie Y, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018;67:1024ŌĆō1032.


3. Nakae H, Tsuda A, Matsuoka T, Mine T, Koga Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol 2016;3:e000109. eCollection 2016.



4. Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res 2012;18:2138ŌĆō2144.



5. Otani K, Tanigawa T, Watanabe T, et al. Microbiota plays a key role in non-steroidal anti-inflammatory drug-induced small intestinal damage. Digestion 2017;95:22ŌĆō28.


6. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164:337ŌĆō340.


7. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533.



8. Lagier JC, Armougom F, Million M, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect 2012;18:1185ŌĆō1193.


9. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007;449:804ŌĆō810.




10. Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018;175:962ŌĆō972; e10.



11. Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 2017;15:630ŌĆō638.



12. Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 2009;137:588ŌĆō597.



13. Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017;77:6777ŌĆō6787.



14. Chen X, Winckler B, Lu M, et al. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS One 2015;10:e0143603.



15. Nasrollahzadeh D, Malekzadeh R, Ploner A, et al. Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Sci Rep 2015;5:8820.




16. Liu Y, Lin Z, Lin Y, et al. Streptococcus and Prevotella are associated with the prognosis of oesophageal squamous cell carcinoma. J Med Microbiol 2018;67:1058ŌĆō1068.


17. Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J 2011;5:574ŌĆō579.



18. Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 2006;103:732ŌĆō737.



19. Kim EJ, Baik GH. Review on gastric mucosal microbiota profiling differences in patients with chronic gastritis, intestinal metaplasia, and gastric cancer. Korean J Gastroenterol 2014;64:390ŌĆō393.


20. Delgado S, Cabrera-Rubio R, Mira A, Su├Īrez A, Mayo B. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol 2013;65:763ŌĆō772.



21. Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018;67:226ŌĆō236.


22. Eun CS, Kim BK, Han DS, et al. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 2014;19:407ŌĆō416.


23. Li TH, Qin Y, Sham PC, Lau KS, Chu KM, Leung WK. Alterations in gastric microbiota after H. pylori eradication and in different histological stages of gastric carcinogenesis. Sci Rep 2017;7:44935.



24. Park JS, Park JE, Oh BS, et al. Trend in the eradication rates of Helicobacter pylori infection over the last 10 years in West Gyeonggi-do, Korea: a single center experience. Korean J Gastroenterol 2017;70:232ŌĆō238.


25. Lu M, Yu S, Deng J, et al. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: a meta-analysis of randomized controlled trials. PLoS One 2016;11:e0163743.



26. Kim JW. The effects of probiotics on the treatment of Helicobacter pylori eradication. Korean J Helicobacter Up Gastrointest Res 2016;16:129ŌĆō133.

27. Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther 2006;23:1077ŌĆō1086.


28. Fang HR, Zhang GQ, Cheng JY, Li ZY. Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials. Eur J Pediatr 2019;178:7ŌĆō16.



29. Wen J, Peng P, Chen P, et al. Probiotics in 14-day triple therapy for Asian pediatric patients with Helicobacter pylori infection: a network meta-analysis. Oncotarget 2017;8:96409ŌĆō96418.



30. Feng JR, Wang F, Qiu X, et al. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol 2017;73:1199ŌĆō1208.



31. Lau CS, Ward A, Chamberlain RS. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori : a meta-analysis. Infect Drug Resist 2016;9:275ŌĆō289; eCollection 2016.



32. McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J 2016;4:546ŌĆō561.


33. Lu C, Sang J, He H, et al. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: a meta-analysis. Sci Rep 2016;6:23522.



34. Gong Y, Li Y, Sun Q. Probiotics improve efficacy and tolerability of triple therapy to eradicate Helicobacter pylori : a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:6530ŌĆō6543; eCollection 2015.


35. Zhang MM, Qian W, Qin YY, He J, Zhou YH. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol 2015;21:4345ŌĆō4357.



36. Lv Z, Wang B, Zhou X, et al. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: a metaanalysis. Exp Ther Med 2015;9:707ŌĆō716.



37. Zhu R, Chen K, Zheng YY, et al. Meta-analysis of the efficacy of probiotics in Helicobacter pylori eradication therapy. World J Gastroenterol 2014;20:18013ŌĆō18021.



38. Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One 2014;9:e111030.



39. Igarashi M, Nakae H, Matsuoka T, et al. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol 2017;4:e000144.



40. Ohtsu T, Takagi A, Uemura N, et al. The ameliorating effect of lactobacillus gasseri OLL2716 on functional dyspepsia in Helicobacter pylori-uninfected individuals: a randomized controlled study. Digestion 2017;96:92ŌĆō102.



41. Takagi A, Yanagi H, Ozawa H, et al. Effects of lactobacillus gasseri OLL2716 on Helicobacter pylori-associated dyspepsia: a multicenter randomized double-blind controlled trial. Gastroenterol Res Pract 2016;2016:7490452.




42. Urita Y, Goto M, Watanabe T, et al. Continuous consumption of fermented milk containing Bifidobacterium bifidum YIT 10347 improves gastrointestinal and psychological symptoms in patients with functional gastrointestinal disorders. Biosci Microbiota Food Health 2015;34:37ŌĆō44.



43. Gomi A, Yamaji K, Watanabe O, et al. Bifidobacterium bifidum YIT 10347 fermented milk exerts beneficial effects on gastrointestinal discomfort and symptoms in healthy adults: a double-blind, randomized, placebo-controlled study. J Dairy Sci 2018;101:4830ŌĆō4841.


44. Kalman DS, Schwartz HI, Alvarez P, Feldman S, Pezzullo JC, Krieger DR. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol 2009;9:85.



45. Ianiro G, Pizzoferrato M, Franceschi F, Tarullo A, Luisi T, Gasbarrini G. Effect of an extra-virgin olive oil enriched with probiotics or antioxidants on functional dyspepsia: a pilot study. Eur Rev Med Pharmacol Sci 2013;17:2085ŌĆō2090.

46. El Aidy S, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol 2015;32:14ŌĆō20.


47. Gu S, Chen D, Zhang JN, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 2013;8:e74957.



48. Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins 1977;14:333ŌĆō341.


49. Uejima M, Kinouchi T, Kataoka K, Hiraoka I, Ohnishi Y. Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol Immunol 1996;40:553ŌĆō560.


50. Watanabe T, Higuchi K, Kobata A, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 2008;57:181ŌĆō187.


51. Watanabe T, Nishio H, Tanigawa T, et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol 2009;297:G506ŌĆōG513.


-
METRICS

-
- 3 Crossref
- 6,277 View
- 225 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Artificial Intelligence in the Analysis of Upper Gastrointestinal Disorders2021 December;21(4)
Helicobacter pylori and Other Gastrointestinal Diseases 2020 March;20(1)
The Diagnosis of Subepithelial Lesions in the Upper Gastrointestinal Tract2011 September;11(2)






