Rapidly Growing, High-Risk Gastrointestinal Stromal Tumor of the Stomach: A Case Report
Article information
Abstract
An increase in the volume of endoscopic procedures performed in recent times has led to increasing detection rates of asymptomatic gastrointestinal subepithelial tumors. However, accurate diagnosis and risk assessment of these tumors preoperatively is challenging. A 70-year-old man patient visited the emergency department for evaluation of melena. Emergency endoscopy revealed an ulcerated subepithelial tumor (8 cm in size) in the gastric cardia and fundus. Computed tomography and upper endoscopy performed at another hospital 6 months earlier were reviewed; the mass showed a significant increase in size (from 2 cm to 8 cm). The patient underwent surgical resection of the mass and was diagnosed with a high-risk gastrointestinal stromal tumor (GIST). In this article, we describe a rare case of a rapidly growing GIST at a rate significantly greater than commonly reported rates.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are non-epithelial entities most commonly observed in the gastrointestinal tract, and account for 1% of all gastrointestinal tumors [1]. The detection of asymptomatic subepithelial tumors of the gastrointestinal tract is increasing due to the increasing number of endoscopy procedures performed [2]. The first-line treatment for GIST without metastasis is surgical resection, and surveillance is recommended for GISTs <2 cm in size [3]. However, this strategy is not always correct because GISTs have malignant potential, and the rate of size increase is not the same in all patients with GIST. We describe a rare case of gastric GIST that exhibited a sudden increase in size in addition to acute gastrointestinal bleeding.
CASE REPORT
A 70-year-old male patient visited a hospital emergency room with chief complaints of melena, epigastric pain, and dizziness. The patient was undergoing treatment for hypertension, hyperlipidemia, and left vertebral artery stenosis, and was taking aspirin. Six months previously, the patient underwent gastroscopy and was diagnosed with a gastric subepithelial tumor, and was examined at another hospital and was advised to under-go observation and follow up rather than surgery. At the time of the emergency room visit, his blood pressure was 118/63 mmHg, with a heart rate of 110 beats/min, a respiratory rate of 20 breaths/min, and a body temperature of 37°C. Melena was confirmed on digital rectal examination. His leukocyte count was 12200/mm3, with a hemoglobin level of 9.3 g/dL, and platelet count of 146000/mm3. The patient’s blood urea nitrogen level was 40.3 mg/dL, with a creatinine level of 1.23 mg/dL. Upper endoscopy revealed a subepithelial tumor, measuring approximately 8 cm in size, in the cardiac area of the stomach, in addition to a 2 cm ulcer within the mass (Fig. 1). Abdominal computed tomography (CT) performed to further characterize the mass revealed a lobulated, contoured solid mass, measuring 8.4 cm in size, in the lesser curvature of the upper body and cardia (Fig. 2). No abnormal lymph nodes or metastases were found in the surrounding tissues. A subepithelial tumor, measuring approximately 2 cm in size, was identified at the same location where previous endoscopic and abdominal CT images from another hospital were obtained and reviewed (Figs. 1 and 2). It was confirmed that there was a rapid increase in the size of the tumor and the patient was transferred to the department of surgery for treatment. Surgery was performed using hand-assisted laparoscopic wedge resection to prevent rupture of the large tumor. The resected mass measured 11.0 cm × 5.5 cm × 5.0 cm, and the resection margins were confirmed to be negative. Microscopy revealed mitosis >5 per 50 high-power fields, and c-kit was positive (Fig. 3). The mass was diagnosed with high-risk GIST, and imatinib (Gleevec; Novartis, Basel, Switzerland) was initiated. The patient discontinued Gleevec due to side effects after 15 months of treatment. However, after an extragastric recurrence occurred in the left subphrenic space, Gleevec was restarted alongside symptomatic care. The patient has been under follow-up and has survived for 41 months after surgery.
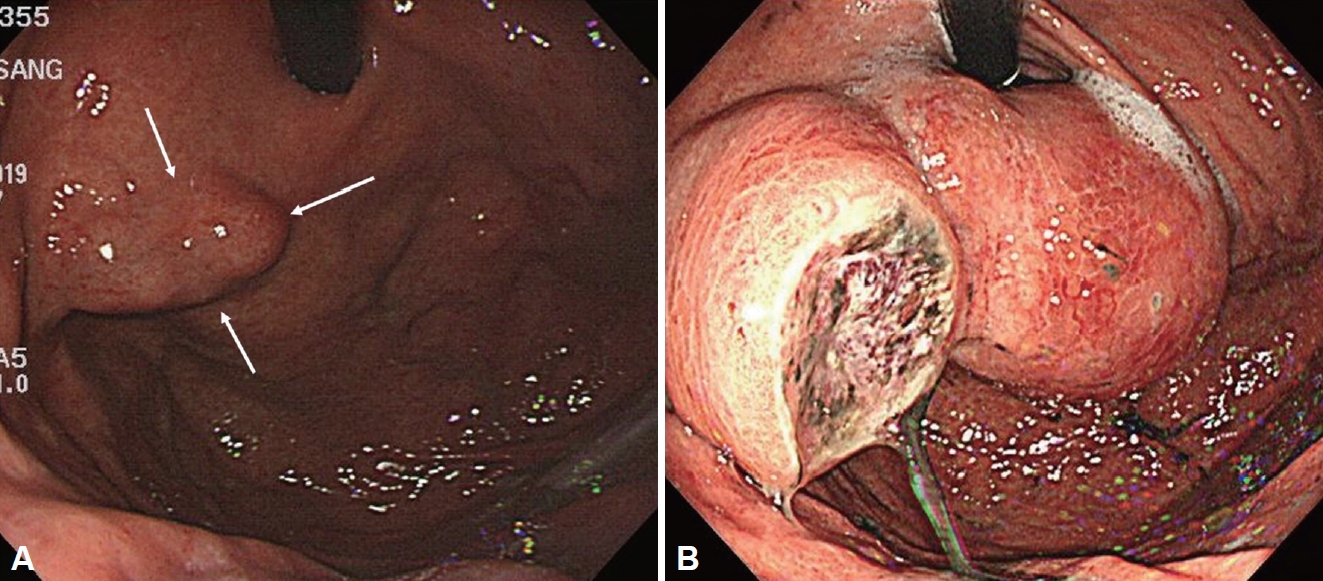
Endoscopy performed 6 months previously showed a 2 cm mass (A, white arrow) at gastric fundus and endoscopy performed at the emergency room revealed an 8 cm mass with ulceration at gastric cardia and fundus (B).
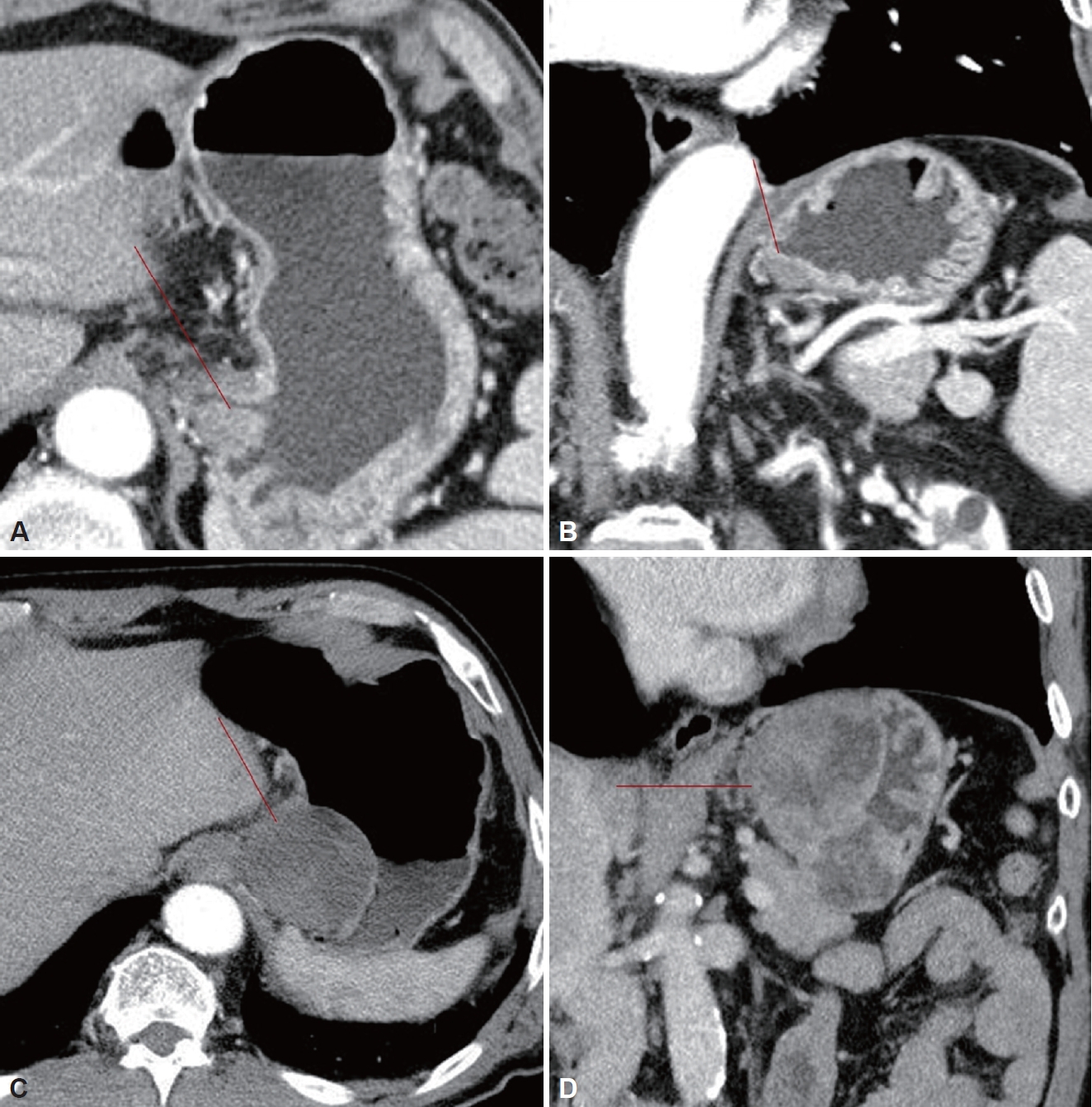
Computed tomography (CT) performed 6 months previously (A: axial image, B: coronal image) showed a 2 cm mass at the fundus (red line) and CT at the emergency room (C: axial image, D: coronal image) showed a large lobulated solid mass (red line) without lymph node metastasis.

The pathologic images of the mass. A: The tumor has a relatively well-defined tumor border (H&E stain, ×40). B and C: The tumor is composed of round cells with variably eosinophilic or clear cytoplasm and showed mitotic figure, consistent with an epithelioid type GIST (H&E stain, ×200, ×400, respectively). D: Immunohistochemistry for c-kit shows diffuse and strong cytoplasmic positivity (×100). GIST, gastrointestinal stromal tumor; H&E, haematoxylin and eosin.
DISCUSSION
According to a recent study, the incidence of GIST is 10–15 per million population, and the stomach is the most frequent site, accounting for approximately 50% of all GISTs [4]. Recently, the detection of GISTs <2 cm is also increasing. The principal treatment for localized GISTs is complete surgical resection, which is recommended for tumors ≥2 cm in size, while observation and follow-up is recommended for smaller GISTs (<2 cm) without signs of malignancy [3]. Even in the most recent guidelines, surgery is not strongly recommended for GISTs measuring less than 2 cm [5,6]. However, such regular follow-up for small GISTs may not be successful. First, some patients may experience stress, depression, and anxiety about the tumor. Second, it can be exceedingly difficult to follow up regularly due to poor compliance [7]. Third, the growth rate of these entities is not necessarily consistent, and rapid growth is possible, as in our case. In a case similar to ours, Tanaka et al. [8] described a gastric GIST that exhibited rapid growth, from 1 cm to 3 cm, and liver metastasis was ultimately reported. The case was also high-risk despite the small size, and there were deletions of five amino acids from codons 554–558 in exon 11 loss of codons 557–558 has been reported to be associated with poor clinical prognosis [9].
The rapid growth of the tumor may have been due to its high-risk characteristics. GISTs are divided into very low, low, intermediate, and high risk groups according to tumor size, mitotic index, and primary site [10]. Unlike size and location, mitosis may not be accurately assessed before surgery because obtaining specimens is difficult using routine biopsy techniques, and tissues obtained via endoscopic ultrasound-fine needle aspiration (EUS-FNA) may be insufficient to assess mitosis [2]. Although a recent mucosal incision-assisted biopsy method provided better diagnostic yields than EUS-FNA, this method required a longer procedure time and may pose a risk for tumor seeding and is only effective for tumors of intraluminal growth [11]. Therefore, treatment decisions usually depend on tumor size without biopsy. However, this strategy may be inadequate.
Some studies have investigated the growth of GISTs. Gao et al. [7] reported that out of 69 EUS suspected GISTs, 16 (23.2%) exhibited significant changes in size. They suggested that GISTs measuring ≥9.5 mm may be associated with significant progression and recommended more short term EUS follow-up (6–12 months) compared with <9.5 mm GIST (2–3 years) after detection. Fang et al. [12] reported that GIST larger than 1.4 cm with irregular margins were associated with significant progression. Kim et al. [13] reported that the growth rate of subepithelial tumors was 0.14, 0.22, and 0.31 mm/month in those <10 mm, 10–20 mm, and >20–30 mm, respectively, and GIST exhibited significant increases in tumor size compared with other benign tumors. Koizumi et al. [14] reported that the doubling time of GIST was 17.2 months, which was short compared with other benign subpeithelial tumors. In particular, for highrisk GISTs, it was 3.9 months, which was shorter than that in the other risk groups. These data suggested that there was a gradual increase in size of GISTs over time. However, our case exhibited significantly faster growth compared to data from these studies.
EUS features can be used as predictive factors for malignancy. High-risk EUS features of GIST include irregular borders, internal heterogeneity, or ulceration, which can aid in treatment decisions for GIST [15]. However, these features could not be used to preoperatively predict the risk for malignancy of mediumsize gastric GISTs in a previous study [16]. Recently, contrast-enhanced harmonic EUS has been used to estimate malignant potential by evaluating the uniformity of the contrast, blood vessels inside the tumor, heterogeneous enhancement, and non-enhancing spots [17].
The timing of surgery is also important. As with other malignant tumors, early treatment can result in a better prognosis. Some authors insist that early diagnosis and early resection may be required because GISTs <2 cm in size could metastasize [2]. Fang et al. [12] reported that the 2 cm criteria for surgery was inadequate for separating the progressive tumors from the stationary ones. Yang et al. [18] even suggested surgical resection of all small gastric GISTs because small GISTs may have malignant potential. However, the risk for surgery also needs to be considered. Therefore, early resection of lesions <2 cm appears to be helpful in relatively young patients who require long-term follow-up.
In conclusion, small GISTs (<2 cm) could potentially exhibit rapid growth. It is necessary to provide appropriate information to patient about the possibility of an increase in size and early resection may be considered in case of GIST.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
None
Authors’ Contribution
Conceptualization: Seung-Woo Lee. Data curation: Sung Jin Lim, Seung- Woo Lee. Formal analysis: Seung-Woo Lee. Investigation: Sung Jin Lim, Seung-Woo Lee. Methodology: Seung-Woo Lee. Project administration: Sung Jin Lim. Resources: Han Mo Yoo, Hae Joung Sul, Seung-Woo Lee. Software: Sung Jin Lim. Supervision: Dong Soo Lee. Validation: Seung- Woo Lee. Visualization: Sung Jin Lim. Writing—original draft: Sung Jin Lim. Writing—review & editing: Seung-Woo Lee. Approval of final manuscript: all authors.
Ethical Statement
This study was approved by the Institutional Review Board of The Catholic University of Korea (No. DC20ZASE0094). Informed consent was obtained from patient for publication of this study.
Acknowledgements
None
