A 10- or 14-day Bismuth-containing Quadruple Therapy as a First-line Helicobacter pylori Eradication Therapy: A Systematic Review and Meta-analysis
Article information
Abstract
Background/Aims
The eradication rate of the first-line standard triple therapy (STT) for Helicobacter pylori (H. pylori) infection has decreased since 2000; therefore, other first-line therapies are required. This study was aimed at investigating the efficacy of bismuth-containing quadruple therapy (PBMT) for first-line H. pylori eradication compared to STT, sequential therapy (SQT), and concomitant therapy (CT).
Materials and Methods
The Ovid-MEDLINE, Koreamed, EMBASE, KMBASE, and Cochrane Library databases were searched from January 2008 to July 2018. All identified randomized controlled trials (RCTs) comparing PBMT and non-PBMT for first-line H. pylori eradication therapy were included in the final analysis.
Results
A total of 3,653 patients from seven RCTs were enrolled. The pooled eradication rates of PBMT by intention-to-treat (ITT) and per-protocol (PP) analyses were 82.1% (95% CI, 68.2~90.8%) and 88.8% (95% CI, 77.1~94.9%), respectively. However, no statistically significant difference was observed in eradication rates of the 10- or 14-day PBMT as compared to 14-day STT, 10-day SQT, and 10-day CT in ITT and PP analyses. PBMT was significantly higher in adverse events than in the other eradication regimens (RR, 1.64; 95% CI, 1.11~2.44). Considerable heterogeneity in adverse events was observed among studies (χ2=88.7; P<0.001, I2=93%).
Conclusions
PBMT can be the first-line treatment for H. pylori eradication in Korea when other first-line options, including STT, SQT, or CT, are unavailable due to their high adverse event rates.
INTRODUCTION
As of 2015, the global prevalence of Helicobacter pylori (H. pylori) was approximately 4.4 billion [1]. This common infection is well known to cause gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer [2]. Therefore, effective eradication therapy is crucial for building public health, particularly in high H. pylori prevalence areas, including Korea.
According to Korean guidelines for the diagnosis and treatment of H. pylori infection, which were revised in 2013, standard triple therapy, comprising proton pump inhibitors (PPIs), amoxicillin, and clarithromycin, is the recommended first-line eradication regimen for H. pylori infection [3]. Unfortunately, the efficacy of the standard triple therapy is declining worldwide, and this tendency is also observed in Korea [4-6]. The H. pylori eradication rates in Korea using standard triple therapy were 84.9~87.5% from 2001 to 2007 and 80.0~81.4% from 2008 to 2010 (P<0.0001), presenting a decreasing trend for the last 10 years [5].
Several recognized guidelines recommended that standard triple therapy, sequential therapy (SQT), concomitant therapy (CT), bismuth-containing quadruple, or non-bismuth-containing quadruple therapies are a recommended first-line treatment option [7,8]. In the aforementioned revised Korean guidelines, when clarithromycin resistance is suspected, bismuth-containing quadruple therapy is used as the first-line therapy; however, it is generally recommended as a second-line regimen to eradicate H. pylori [3].
Bismuth-containing quadruple therapy is an effective regimen for H. pylori eradication. However, the efficacy of bismuth-containing quadruple therapy as a first-line treatment has depended on the type of eradication regimens and duration of therapies in two network meta-analyses of the randomized controlled trials (RCTs) [9,10]. With this background, a meta-analysis was conducted to evaluate the efficacy and safety of bismuth-containing quadruple therapy for first-line H. pylori eradication and compare it to standard triple therapy, SQT, and CT, which are commonly used as first-line eradication therapies.
MATERIALS AND METHODS
1. Search strategy
We searched Ovid-MEDLINE, KoreaMed, EMBASE, KMBASE, and Cochrane Library from January 2008 to July 2018. The exploring key words were “Helicobacter pylori or Helicobacter infection” and “bismuth based quadruple therapy, bismuth therapy, or bismuth treat”. Languages were limited to English and Korean. The reporting of the current study follows the PRISMA guidelines [11].
2. Study selection
Studies were included in the meta-analysis if the following criteria were met: 1) RCTs; 2) bismuth-containing quadruple therapy as a first-line eradication therapy; 3) H. pylori infection was identified by at least one of the following modalities: rapid urease test, urea breath test, histology, or culture; 4) successful eradication of H. pylori infection was clarified with previous mentioned modalities; and 5) bismuth-containing quadruple therapy consisting of PPIs, bismuth compounds, metronidazole, and tetracycline.
Non-RCTs, studies with second-line treatment using bismuth-containing quadruple therapy, bismuth-containing regimens including PyleraⓇ (Allegran, Dublin, Ireland), control groups consisting of the same regimens as the intervention group with different duration or different doses, reviews, case reports, letters, editorials, and abstracts were excluded.
Two investigators (S.E.K. and H.K.J.) separately reviewed the results of the search, and selected the included studies. Disagreement was resolved by consensus.
3. Data extraction
The following information was collected from the enrolled studies: 1) name of the first author, year of publication, country, and trial types; 2) number of subjects in each study according to therapeutic regimens, mean age, therapeutic regimens, duration of regimens, and funding; 3) diagnostic methods of confirming H. pylori infection and eradication, and eradication follow-up period; and 4) number of subjects who were successfully treated in each regimen, eradication rates of both intention-to-treat (ITT) and per-protocol (PP) analyses, and adverse events.
4. Risk of bias
Two investigators (S.E.K. and H.K.J.) independently assessed the potential bias of enrolled studies using Cochrane Handbook for Systemic Reviews in Intervention [12]. The assessment tool of Cochrane Collaboration was categorized as randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome data.
5. Statistical analysis
All statistical analyses were performed with Review Manager version 5.3 software (Revman; The Cochrane Collaboration Oxford, UK). The pooled eradication rate with 95% CI in bismuth-containing quadruple therapy and non-bismuth-containing quadruple therapy was calculated. Thereafter, subgroup analyses were conducted according to the eradication regimens. The assessments of the subgroup analyses and adverse events were expressed as the RR with 95% CI. We investigated heterogeneity between studies by using I2 statistics. We considered I2 value of 25%, 50%, and 75% to be low, moderate, and high degrees of heterogeneity, respectively. When high heterogeneity occurred, we calculated RRs using random-effect models. We used both ITT and PP analyses for clinical outcomes. A P<0.05 was suggested as being statistically significant.
RESULTS
1. Study selection
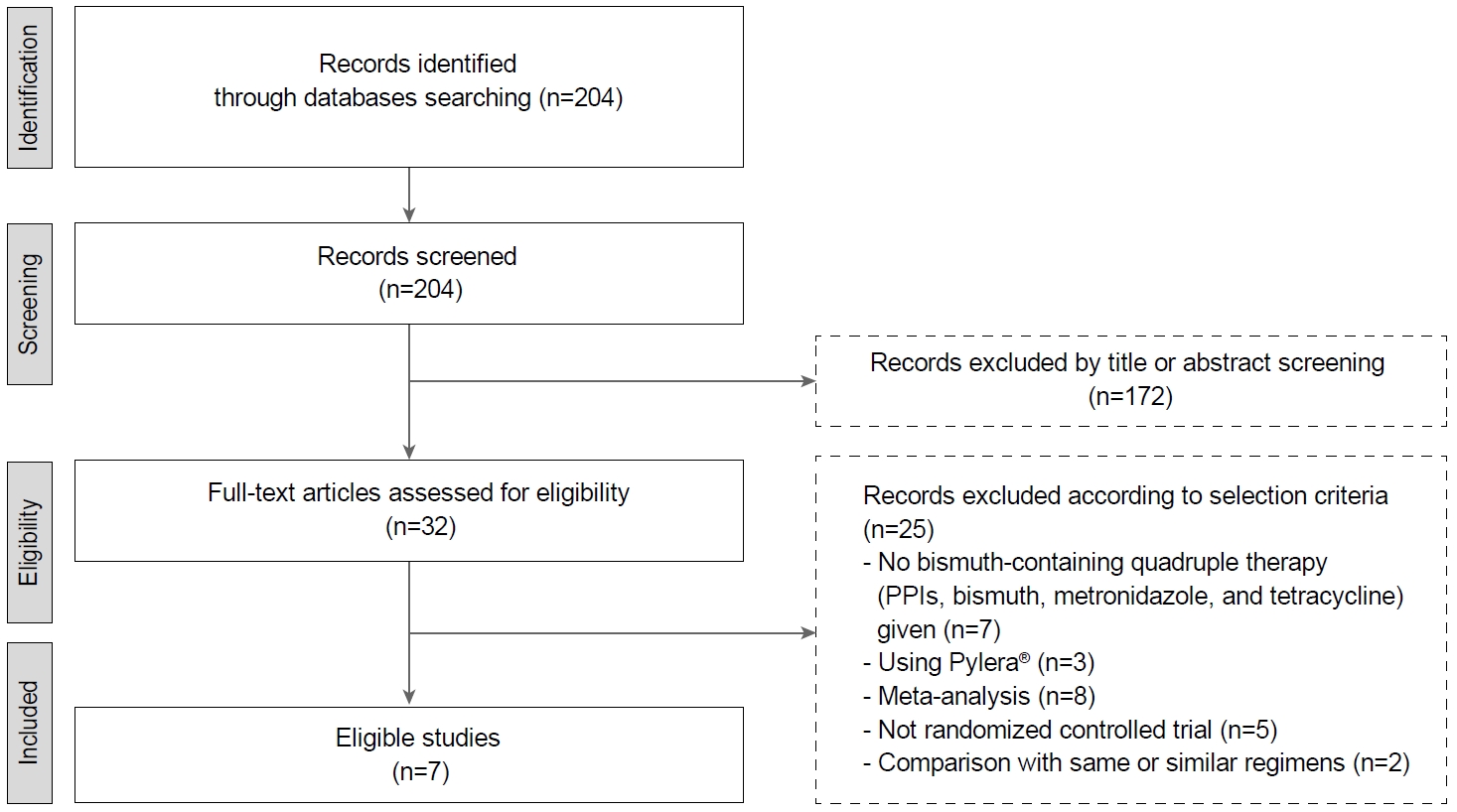
A total of 204 studies were reviewed. Fig. 1 shows the flow diagram of the literature search. Subsequently, we excluded 172 studies after reviewing the title or abstract, and we reviewed full articles or abstracts for 32 studies. Among them, 25 studies were excluded according to the selection criteria. Finally, seven RCTs with 3,653 patients were enrolled for meta-analysis [13-19]. The study design of five trials was multiple arms; therefore, the data of the groups that accorded with the inclusion criteria were selected and included for analysis.
2. Characteristics of studies and risk of bias
Table 1 presents the main characteristics of the studies. All studies used a first-line bismuth-containing quadruple therapy consisting of PPIs (pantoprazole, rabeprazole, or lansoprazole), bismuth compounds (bismuth subcitrate, bismuth tripotassium dicitrate, bismuth potassium citrate, colloidal bismuth subcitrate, or bismuth subsalicylate), metronidazole, and tetracycline (PBMT). Patients who received PBMT were 1,206, and 2,447 patients were treated with non-PBMT regimens. Among the non-PBMT regimen groups, 753 patients of three trials in standard triple therapy group, 230 patients in two trials in the SQT group, and 770 patients of three trials in the CT group were eligible to subgroup analysis for eradication efficacy. The risks of bias assessment in seven trials are shown in Supplementary Table 1.
3. Eradication rates of PBMT
The pooled eradication rates of PBMT were 82.1% (95% CI, 68.2~90.8%) in ITT analysis and 88.8% (95% CI, 77.1~94.9%) in PP analysis. The pooled eradication rates of non-PBMT were 77.7% (95% CI, 62.7~88.0%) and 83.3% (95% CI, 68.0~92.1%) in ITT and PP analyses, respectively. In terms of the treatment duration, the pooled eradication rates of 10-day PBMT were 79.6% (95% CI, 47.4~94.4%) and 88.6% (95% CI, 54.7~98.0%) in ITT and PP analyses, respectively. The pooled eradication rates of 14-day PBMT were 83.9% (95% CI, 66.9~93.1%) in ITT analysis and 88.9% (95% CI, 77.2~95.0%) in PP analysis.
4. Eradication rates between PBMT and standard triple therapy
Of three trials, 759 patients were prescribed PBMT for 10 or 14 days, and 753 patients received standard triple therapy for 14 days (Fig. 2). When we compared eradication rates between 10- or 14-day PBMT and 14-day standard triple therapy, the differences were not statistically significant in ITT and PP analyses (ITT analysis: RR, 1.28; 95% CI, 0.97~1.70; P=0.08; PP analysis: RR, 1.37; 95% CI, 0.95~1.99; P=0.10). However, heterogeneity among the studies was high in the ITT and PP analyses (ITT analysis: χ2=10.14, P=0.006, I2=80%; PP analysis: χ2=20.95, P<0.001, I2=90%).
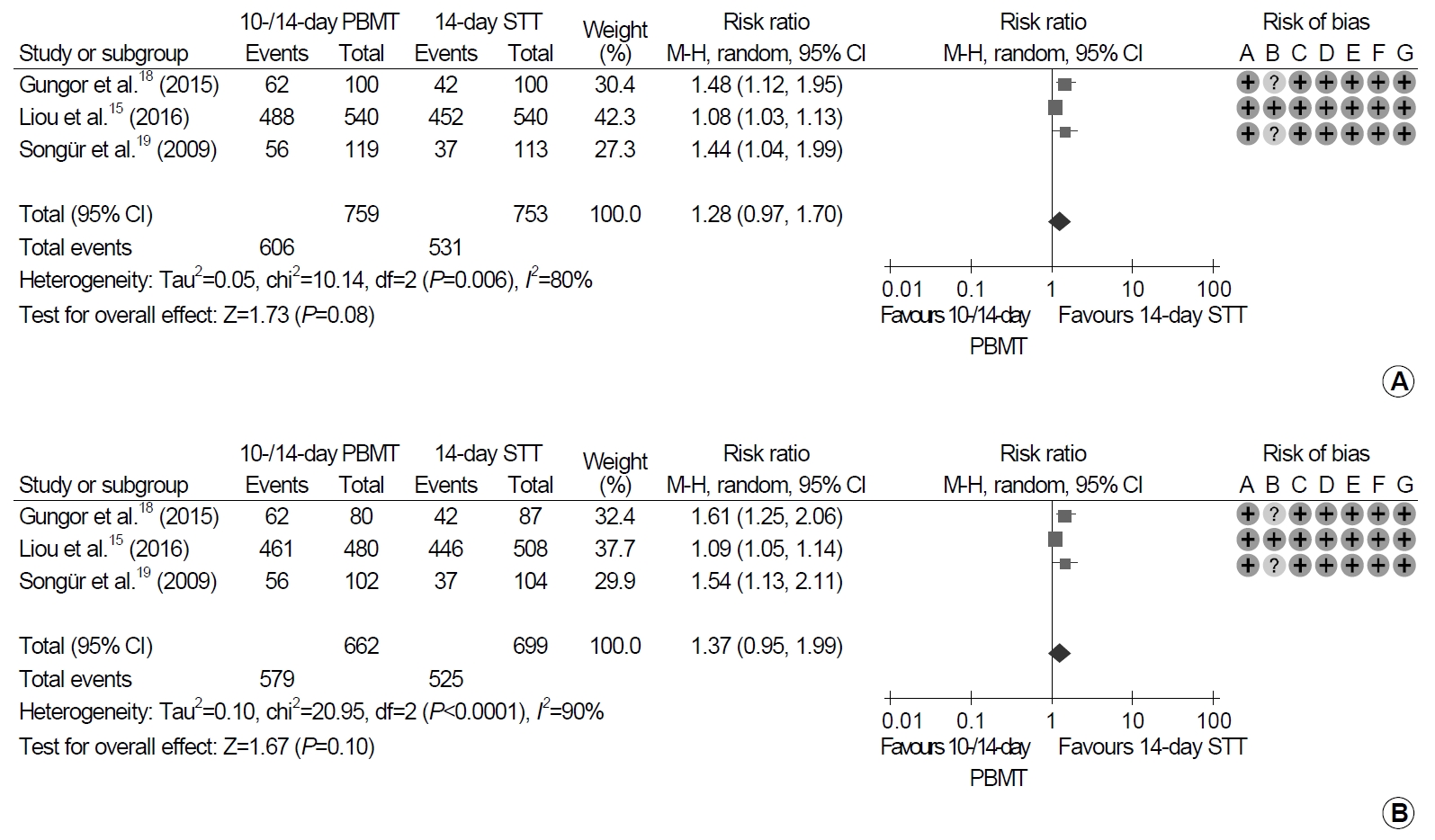
Forest plot of Helicobacter pylori eradication of 10- or 14-day bismuth-containing quadruple therapy (PMBT) vs. 14-day standard triple therapy (STT) as the first-line therapy. (A) Intention-to-treat analysis. (B) Per-protocol analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. M-H, Mantel-Haenszel test.
5. Eradication rates between PBMT and SQT
Each of 230 patients were treated with 10- or 14-day PBMT and 10-day SQT (Fig. 3). Eradication rates between 10- or 14-day PBMT and 10-day SQT were not significantly different in ITT and PP analyses (ITT analysis: RR, 0.96; 95% CI, 0.83~1.12; P=0.59; PP analysis: RR, 0.99; 95% CI, 0.93~1.05; P=0.67). Heterogeneity between the studies was moderate in ITT analysis (χ2=2.08, P=0.15, I2=52%), and low in PP analysis (χ2=0.22, P=0.64, I2=0%).
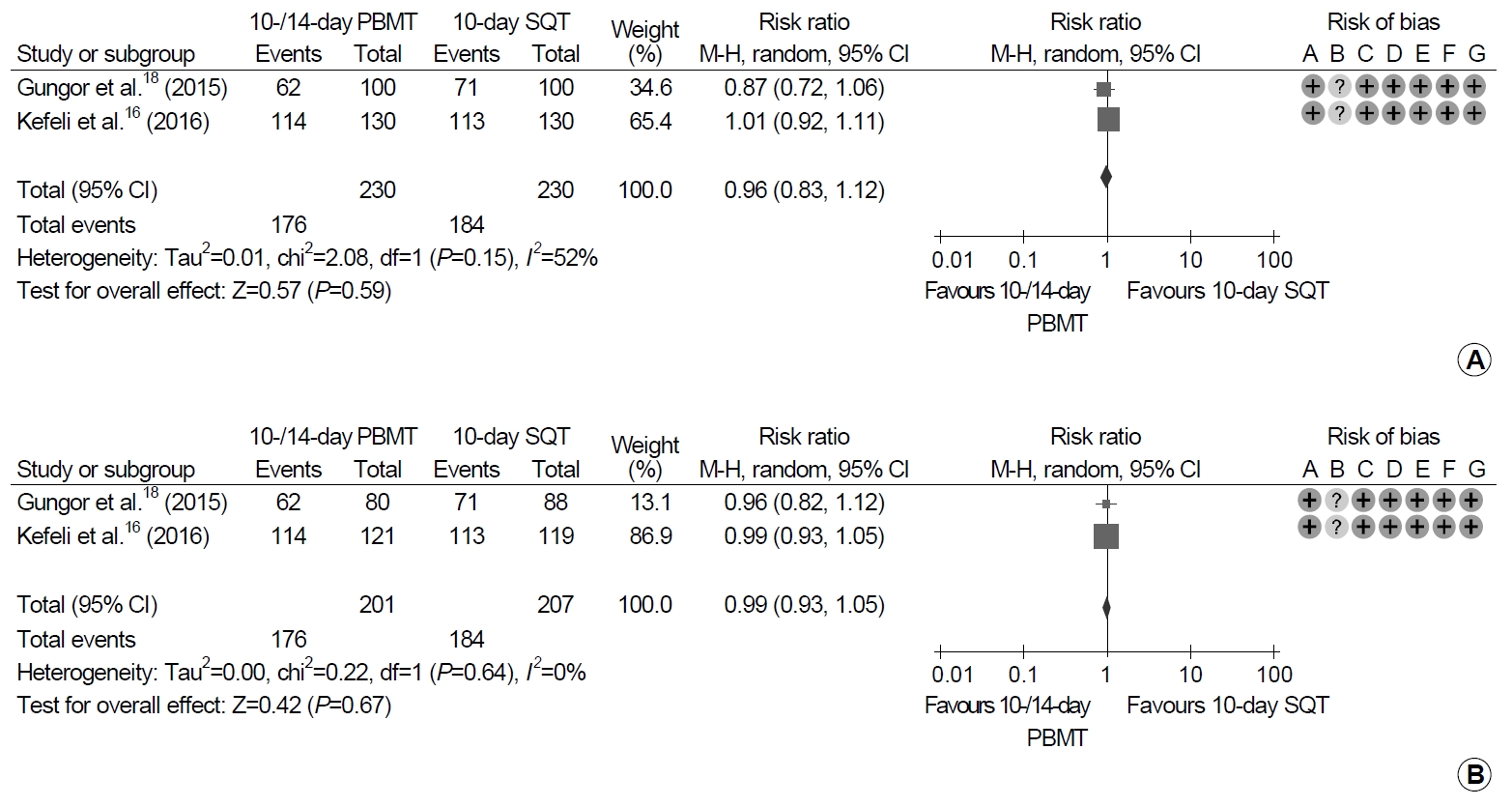
Forest plot of Helicobacter pylori eradication of 10- or 14-day bismuth-containing quadruple therapy (PBMT) vs. 10-day sequential therapy (SQT) as the first-line therapy. (A) Intention-to-treat analysis. (B) Per-protocol analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. M-H, Mantel-Haenszel test.
6. Eradication rates between PBMT and CT
Each of 770 patients received PBMT for 10 or 14 days and CT for 10 days (Fig. 4). There were no significant differences between 10- or 14-day PBMT and 10-day CT groups in either analyses (ITT analysis: RR, 1.01; 95% CI, 0.93~1.10; P=0.83; PP analysis: RR, 1.01; 95% CI, 0.95~1.07; P=0.83). Moderate heterogeneity in the studies was revealed in both the ITT and PP analyses (ITT analysis: χ2=4.57, P=0.10, I2=56%; PP analysis: χ2=5.51, P=0.06, I2=64%).
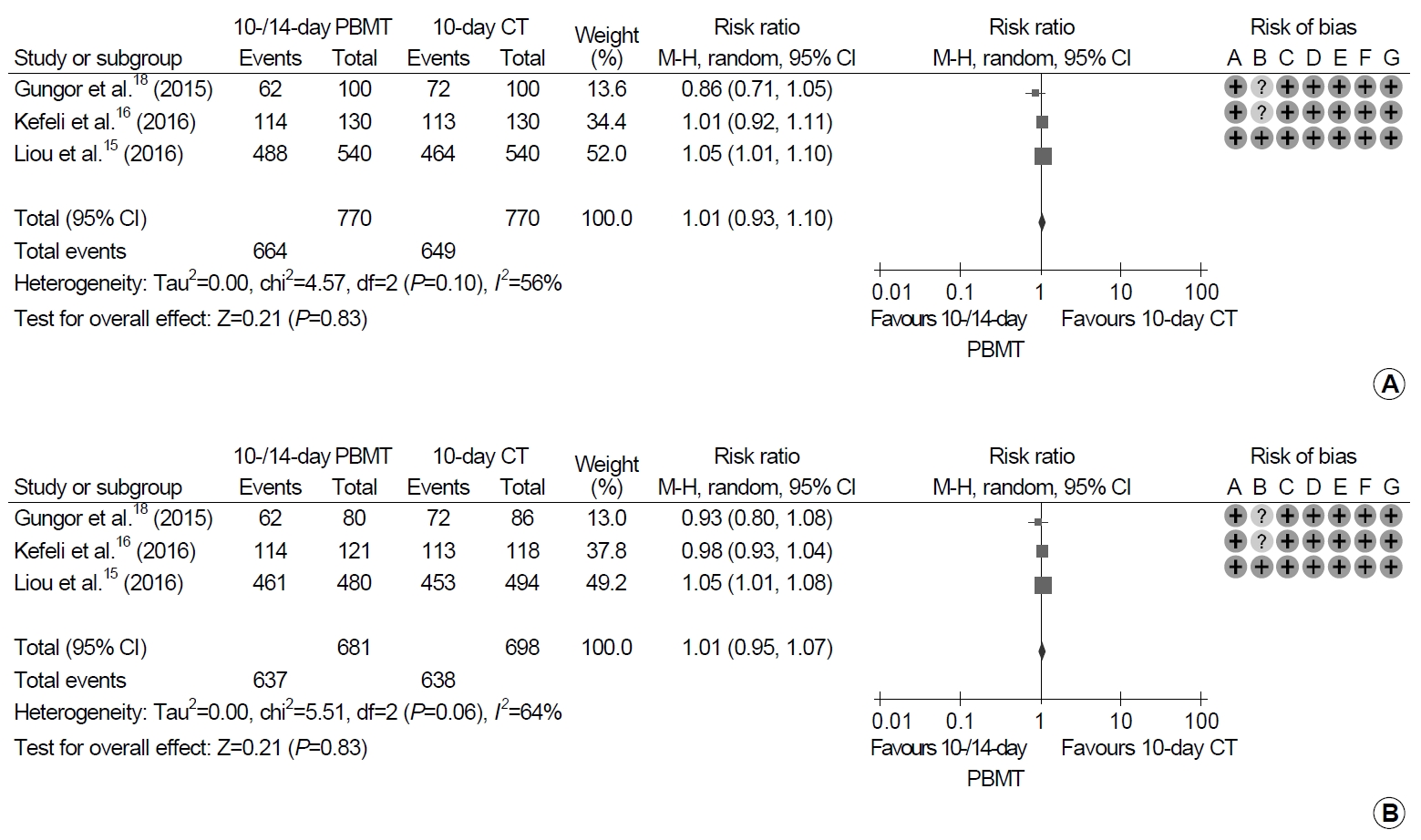
Forest plot of Helicobacter pylori eradication with 10- or 14-day bismuth-containing quadruple therapy (PBMT) vs. 10-day concomitant therapy (CT) as the first-line therapy. (A) Intention-to-treat analysis. (B) Per-protocol analysis. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. M-H, Mantel-Haenszel test.
7. Adverse events
All seven trials identified adverse events with treatment. With regard to the duration of the treatment, the pooled adverse event rate of 10-day PBMT were 44.6% (95% CI, 22.5~69.1%) and those of 14-day PBMT were 55.9% (95% CI, 27.6~80.8%). The meta-analysis of these trials revealed that the frequency of adverse events was significantly higher in patients with PBMT than in those with non-PBMT, and the pooled RR for adverse events was 1.64 (95% CI, 1.11~2.44; P=0.01) (Fig. 5). However, significant heterogeneity in adverse event was observed in the trials (χ2=88.7, P<0.001, I2=93%).
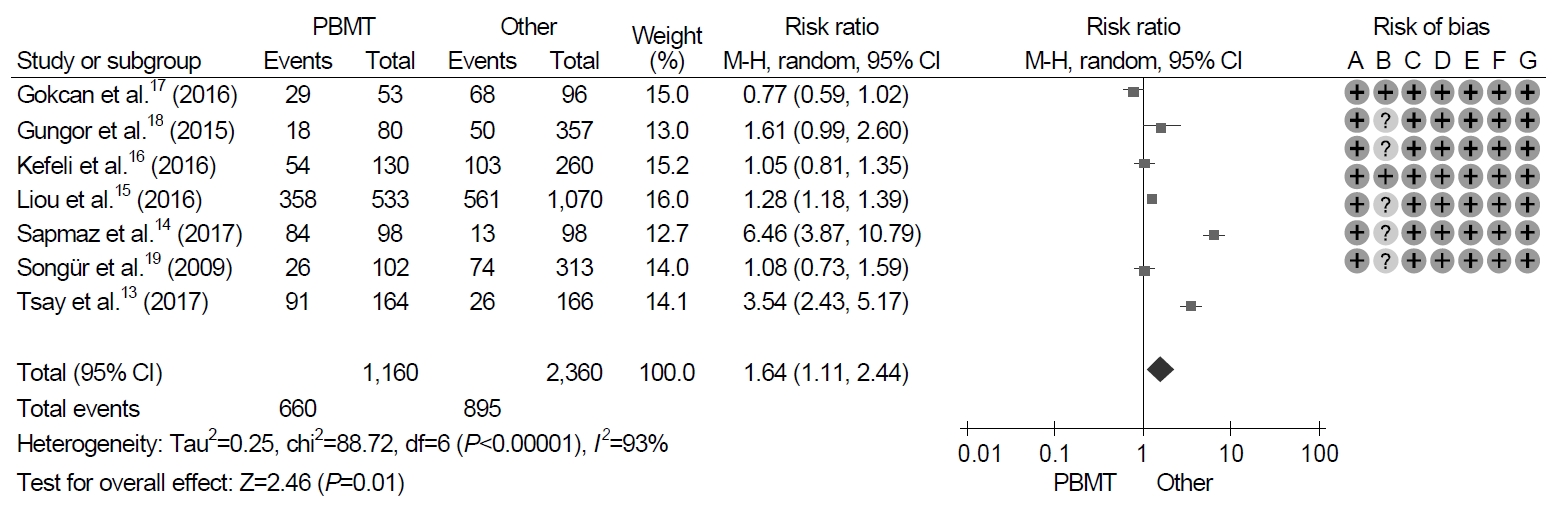
Forest plot of Helicobacter pylori eradication with bismuth-containing quadruple therapy (PBMT) vs. non-bismuth-containing quadruple therapy as the first-line therapy. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias. M-H, Mantel-Haenszel test.
DISCUSSION
Eradication rate of H. pylori is closely related to antibiotics resistance. Among them, the resistance of clarithromycin reduces the success rate of H. pylori eradication. A recent meta-analysis reported that the pooled RR of the eradication rate was 0.682 (95% CI, 0.636~0.731) in patients with H. pylori strains sensitive versus resistant to clarithromycin, whereas the pooled RR of the eradication rate was 0.843 (95% CI, 0.810~0.877) and 0.794 (95% CI, 0.669~0.941) in patients with H. pylori strains sensitive versus resistant to metronidazole and levofloxacin, respectively [20]. Compared to other antibiotics, clarithromycin had the most pervasive influence in eradication rates.
High clarithromycin resistance areas are commonly defined as the clarithromycin resistance rate of H. pylori, which is at least 15% [7]. Clarithromycin resistance has increased during the last decade in Korea [21], and recent Korean studies still presented that clarithromycin resistance rates were 17.8~31.0% [22,23]. At least for first-line H. pylori eradication therapy, successful eradication rates should be more than 80% in ITT analysis and more than 90% in PP analysis [24]. Established guidelines recommended that bismuth-containing quadruple or non-bismuth-containing quadruple therapies are appropriate as the first-line H. pylori eradication therapy in areas of high clarithromycin resistance [7,8]. This meta-analysis revealed that the pooled eradication rate for PBMT as a first-line eradication therapy was 82.1% and 88.8% in ITT and PP analyses. Although the eradication rate in PP analysis did not reach 90%, the effect of PBMT was assumed to be sufficient because the eradication rate was almost 90%.
Nonetheless, when we compared the eradication rates between PBMT and other widely used first-line eradication regimens, there were no statistically significant differences of eradication rates in 10- or 14-day PBMT with 10-day SQT (RR, 0.99; 95% CI, 0.93~1.05), 10-day CT (RR, 1.01; 95% CI, 0.95~1.07), or even 14-day standard triple therapy (RR, 1.37; 95% CI, 0.95~1.99). Our results indicate that 14-day standard triple therapy, 10-day SQT, and 10-day CT are not inferior to 10- or 14-day PBMT.
Duration of treatment can affect the H. pylori eradication rate, and regimens of longer duration had higher eradication rates than did regimens of shorter duration [10]. In terms of standard triple therapy, 14-day standard triple therapy (OR, 1.72; 95% CI, 1.37~2.17) and 10-day standard triple therapy (OR, 1.32; 95% CI, 1.04~1.69) showed a higher eradication rate than did 7-day standard triple therapy [10]. Several meta-analyses have demonstrated similar results for standard triple therapy [7,9,25]. Although the eradication rates of standard triple therapy tend to decrease, 14-day standard triple therapy could be an acceptable regimen, since it has a higher therapeutic effect than does standard triple therapy with a shorter duration.
The results obtained in our study comparing PBMT and SQT are consistent with previous meta-analyses. According to the systematic review and meta-analysis study which analyzed 46 RCTs with 13,532 patients, the overall eradication rate of SQT was 84.3% (95% CI, 82.1~86.4%) [26]. SQT was superior to 7-day standard triple therapy (RR, 1.21; 95% CI, 1.17~1.25) and 10-day standard triple therapy (RR, 1.11; 95% CI, 1.04~1.19) [26]. However, SQT was not superior to 14-day standard triple therapy (RR, 1.00; 95% CI, 0.94~1.06) and 10- or 14-day of bismuth-containing quadruple therapy (RR, 1.01; 95% CI, 0.95~1.06) [26].
Two prospective studies comparing PBMT and CT as first-line treatment were recently published, and CT showed high eradication rates in both studies. A study from Spain reported that the eradication rate was 98.0% (95% CI, 94~100%) in a 14-day CT group and 94.4% (95% CI, 88.1~100%) in a 10-day PBMT group at ITT analysis (P=0.346) [27]. There was no significant difference in adverse events rates between CT (56.0%) and PBMT (46.3%) (P=0.323) [27]. The other was a Korean study that conducted a prospective open-label randomized trial comparing 14-day modified PBMT and 14-day CT [28]. The ITT eradication rate was 88.2% in a modified PBMT group and 79.4% in a CT group (P=0.162) [28]. PP eradication rates were 98.4% and 93.1% in the modified PBMT and CT groups, respectively (P=0.199) [28]. Adverse events rates were higher in CT (51.5%) than in modified PBMT (33.8%) patients (P=0.037) [28]. The adverse event rates of both studies were higher in the CT group than in the PBMT group; however, it should be considered that the duration of CT is longer than that of PBMT in the Spanish study, and the Korean study used modified PBMT that was prescribed PBMT twice a day. A meta-analysis of 19 clinical trials with 2,070 patients reported that a mean ITT cure rate was 88% (95% CI, 85~91%) for CT [29].
It is already known that adverse events of PBMT are common. The adverse events rates of PBMT typically arise in 50% or more [30]. The rates of adverse events were 67% (358/533) in 10-day PBMT, 58% (309/535) in 10-day CT, and 47% (252/535) in 14-day standard triple therapy groups in a multi-center, randomized trial from Taiwan [15]. Although we observed considerable heterogeneity among trials, the adverse events of PBMT were significantly higher than in other eradication therapies (RR, 1.64; 95% CI, 1.11~2.44) in the current meta-analysis. A recent network meta-analysis including 35 studies with 10,860 patients for demonstrating adverse events reported that PBMT showed more adverse events than did SQT and CT [31]. Compared to 14-day PBMT, 14-day SQT (OR, 0.42; 95% CI, 0.22~0.80), 10-day SQT (OR, 0.49; 95% CI, 0.31~0.78), and 10- or 14-day CT (OR, 0.57; 95% CI, 0.37~0.89) therapies were significantly lower in the adverse events rates [31]. There was no significant difference in rates of adverse events between PBMT and standard triple therapy groups in other meta-analyses; however, the overall incidence of adverse events was 46% in PBMT and 46.3% in the standard triple therapy groups [32]. Therefore, the adverse events of PBMT are an issue to consider in clinical practice when using PBMT regimens.
Another problem to take account of in prescribing PBMT is rescue therapy related. It is difficult to decide on a rescue therapy after failure of PBMT. The Maastricht V/Florence consensus report recommended a quinolone-containing triple or quadruple therapy in patients who failed with PBMT [7]. In high quinolone-resistance areas, it is recommended to use the combination of bismuth with other antibiotics [7]. The revised Korean guideline recommended a rescue regimen containing two or more antibiotics that had not been included in PBMT in patients with eradication failure for PBMT [3]. Given the quinolone resistance to H. pylori, the resistance rates of both levofloxacin and moxifloxacin revealed a tendency to increase time-dependently (4.7~28.1%, P=0.002) in Korea [21]. A recent Korean study showed that the resistance rates of both levofloxacin and ciprofloxacin were 37.0% [33]. Another study from Korea compared 7-day PBMT with 14-day moxifloxacin triple therapy as a second-line H. pylori eradication therapy [34]. Eradication rates were 93.6% (95% CI, 91.0~95.9%) and 73.8% (95% CI, 63.1~84.6%) in 7-day PBMT and 14-day moxifloxacin triple therapy groups, respectively (P<0.001) [34]. Despite the shorter duration of PBMT, it exhibited a higher eradication rate than did the longer duration of moxifloxacin triple therapy. H. pylori culture and antimicrobial susceptibility testing are preferred methods to be used after eradication failure of PBMT regimens [7]. Actually, few laboratories have been installed with these systems in Korea. Thus, though PBMT is a valuable treatment for H. pylori infection, PBMT as a first-line therapy could be recommended for patients with an allergy to penicillin, with a clarithromycin-resistant H. pylori infection, or with a history of previous macrolide use.
This study has several limitations. First, only a few trials were included in our analysis, and heterogeneities among studies were generally moderate or high. Therefore, this should be considered when interpreting our results. Second, studies from Korea could not be analyzed because there are no randomized controlled Korean studies on this subject. However, several Turkish and Taiwanese studies were included in this analysis. Turkey belonged to areas of high H. pylori prevalence (77.2%) [1] and high clarithromycin resistance (28%) [35]. The prevalence of H. pylori infection in Taiwan was 53.9% [1] and clarithromycin resistance was 26% [35]. Therefore, these results could reflect those in Korea.
In conclusion, PBMT can be a first-line treatment for H. pylori eradication when other first-line options are unavailable, because of its high adverse events rate and its potential for use as rescue therapy. Further well-designed studies are needed to prove the efficacy of PBMT for first-line H. pylori eradication in Korea.
Notes
Sung Eun Kim is an editorial board member of the Journal but did not involve in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Supplementary Materials
Risk of Bias Assessment for the Studies