Gastric Cancer Showing Rapid Recurrence and Progression: A Case of Gastric Adenocarcinoma With Enteroblastic Differentiation
Article information
Abstract
Gastric adenocarcinoma with enteroblastic differentiation (GAED) is rare and its clinicopathological characteristics are not well documented. However, reports indicate that it exhibits more aggressive characteristics, including lymph node metastasis or liver metastasis, than a conventional gastric adenocarcinoma. Herein, we report a case of GAED with rapid recurrence and disease progression. A 55-year-old male, diagnosed with gastric cancer (GC), demonstrated initial endoscopic findings suggestive of advanced GC. He underwent curative resection since there was no evidence of lymph node or distant metastases. The disease was reported as an early GC that was confined to the submucosal layer, without evidence of lymph node metastasis in the final pathological results. However, six months after surgery, multiple hepatic metastases were found during abdominal computed tomography; the pathological results were consistent with metastasis from the GC. Immunohistochemistry of the primary carcinoma pathological specimens showed positive results for alpha-fetoprotein and sal-like protein 4, suggesting enteroblastic differentiation, which is thought to be associated with rapid recurrence and disease progression.
INTRODUCTION
Gastric adenocarcinoma with enteroblastic differentiation (GAED), also known as clear cell gastric carcinoma, is rare, and its clinicopathological characteristics are not well known [1,2]. To summarize the results of the studies so far, GAED is known to be associated with the production of alpha-fetoprotein (AFP) in the blood and tissue [3]; however, not all GAEDs are positive for AFP and connected to sal-like protein 4 (SALL4) and glypican 3, the fetal gastrointestinal markers [3]. Clinically, it exhibits aggressive characteristics compared to conventional gastric adenocarcinoma (CGA), which has a higher risk of lymphatic invasion [4] and liver metastasis [5]. Herein, we reported a case of GAED with rapid recurrence and progression after radical resection.
CASE REPORT
A 55-year-old male patient with a history of cerebral infarction (13 years prior to the diagnosis of gastric cancer [GC]), hypertension, and dyslipidemia was referred from a local clinic because of a suspected malignant lesion detected on screening upper endoscopy. Endoscopically, a mass lesion approximately 5 cm in size with central ulceration and thickening of the surrounding mucosal folds was observed on the anterior wall of the midbody, which was grossly consistent with Borrmann type III advanced gastric cancer (AGC) (Fig. 1). Abdominal computed tomography (CT) showed thickening of the stomach wall without enlarged lymph nodes or distant metastasis (Fig. 2); therefore, the patient was clinically diagnosed with resectable AGC (cT2-3, N0, M0). Other preoperative evaluations, including laboratory tests, were within normal ranges.
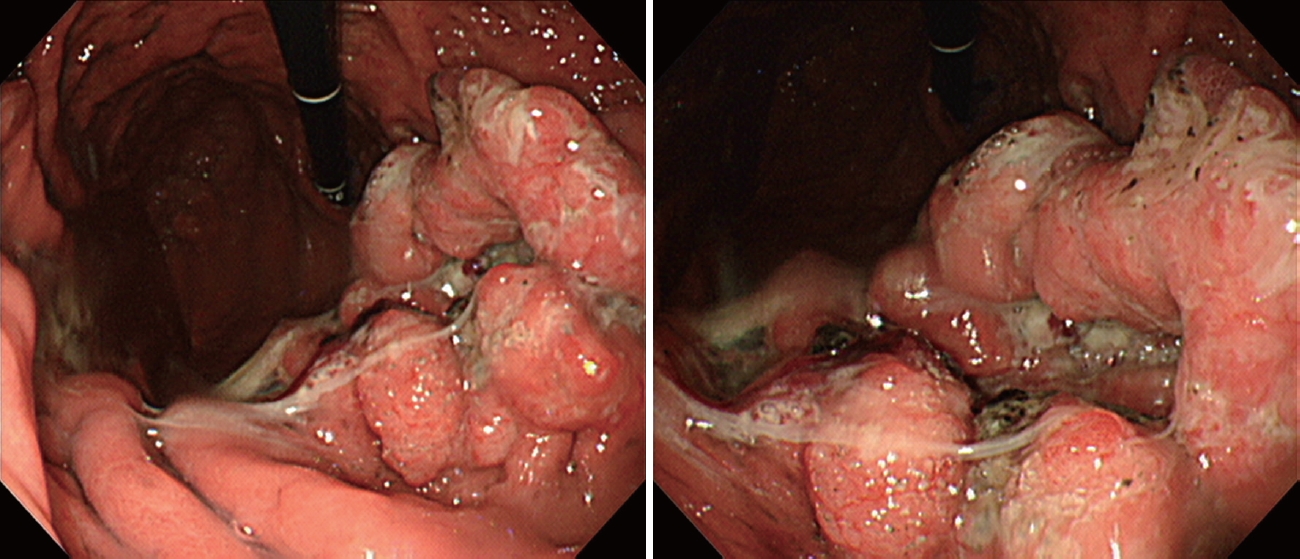
Initial esophagogastroduodenoscopy. The lesion, a mass approximately 5 cm in size, with central ulceration and thickening of the surrounding mucosal folds on the anterior wall of the midbody.
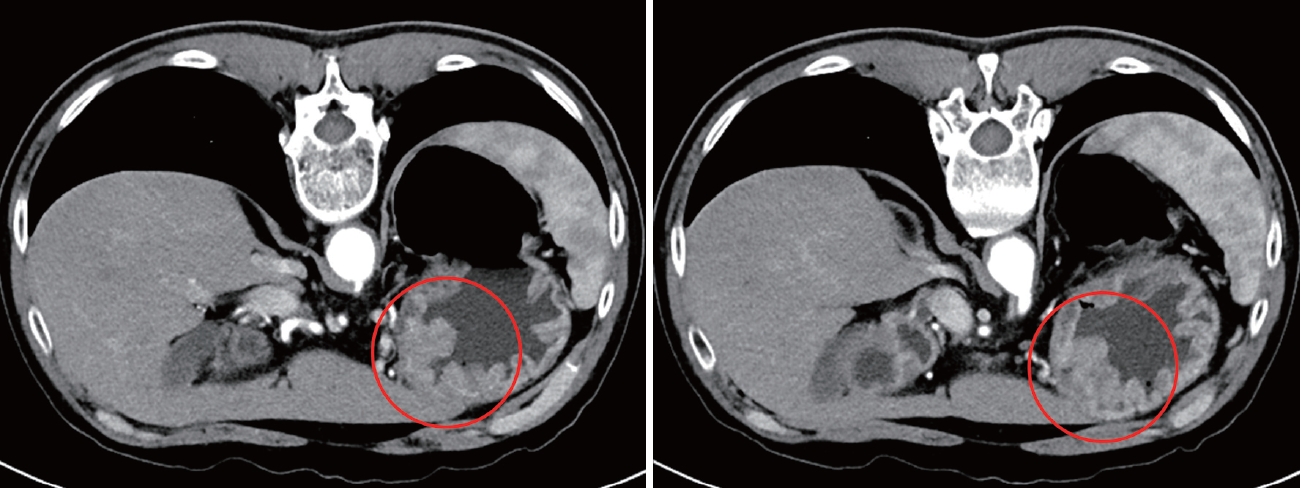
Initial abdomen-pelvis CT. Wall-thickening lesions of T2 or T3 were observed on CT scan (red circles), and no lymph node metastasis or distant metastasis was found. CT, computed tomography
Surgical treatment was decided based on the above findings, and laparoscopy-assisted total gastrectomy with Roux-en-Y reconstruction was performed one month after the diagnosis. The operation and postoperative recovery processes were as usual, and the patient was discharged one week after surgery. The pathology report revealed moderately differentiated gastric adenocarcinoma that was 7.6×5.4×1.5 cm in size, which corresponded to the intestinal type in the Lauren classification. The final staging was early gastric cancer (EGC) (pT1b, N0, stage IA according to the American Joint Committee on Cancer 8th Edition [6]) since the tumor invaded the submucosa and corresponded to T1b (depth from the mucularis mucosa 2000 μm), and there were no lymph node metastasis (Fig. 3). The follow-up was performed without further treatment.

Initial histologic findings. A: Cross section of the surgical specimen. B: Tumor infiltration to the submucosal layer was confirmed (H&E, ×10). C: Tubulopapillary growth pattern was mainly observed, and the tumor was initially diagnosed as tubuloadenocarcinoma with moderate differentiation (H&E, ×200). H&E, hematoxylin and eosin.
During follow-up, multiple hepatic metastases were observed on abdominal CT performed six months after surgery (Fig. 4A and B). Positron tomography (PET) (Fig. 4C and D) scan and percutaneous biopsy for metastatic lesions of the liver were performed for the possibility of metastasis from other organs. Histological finding was consistent with hepatic metastasis, not originated from the liver (Fig. 5A), and PET scan was negative for other suspected primary lesions. A thorough and detailed pathologic review of the initial surgical specimen found a small portion of high-grade changes in the nucleus with a clear cytoplasm suggesting enteroblastic differentiation similar to histologic finding of liver metastasis, which was not found in the initial representative section of the lesion (Fig. 5B). Additionally, immunohistochemistry performed on pathological specimens of the primary carcinoma showed positive results for SALL4 and AFP, suggesting GAED (Fig. 5C and D). The staining for glypican 3 was negative (Fig. 5E).
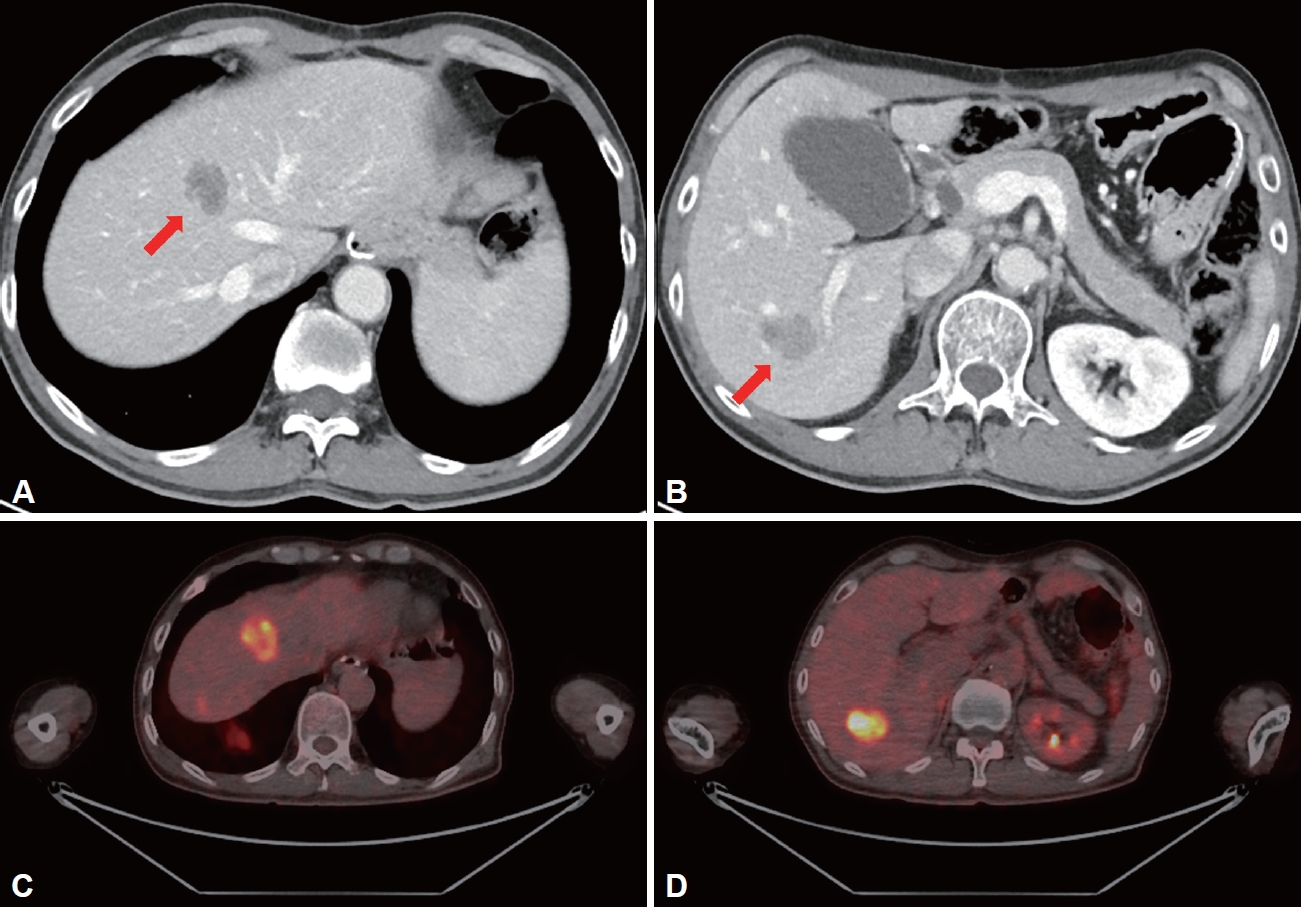
Abdomen-pelvis CT and PET-CT at the 6th month of follow-up. Multiple hepatic metastases (red arrows) were found on CT scan (A and B) and PET-CT scan six months after surgery (C and D). CT, computed tomography; PET, positron-emission tomography.
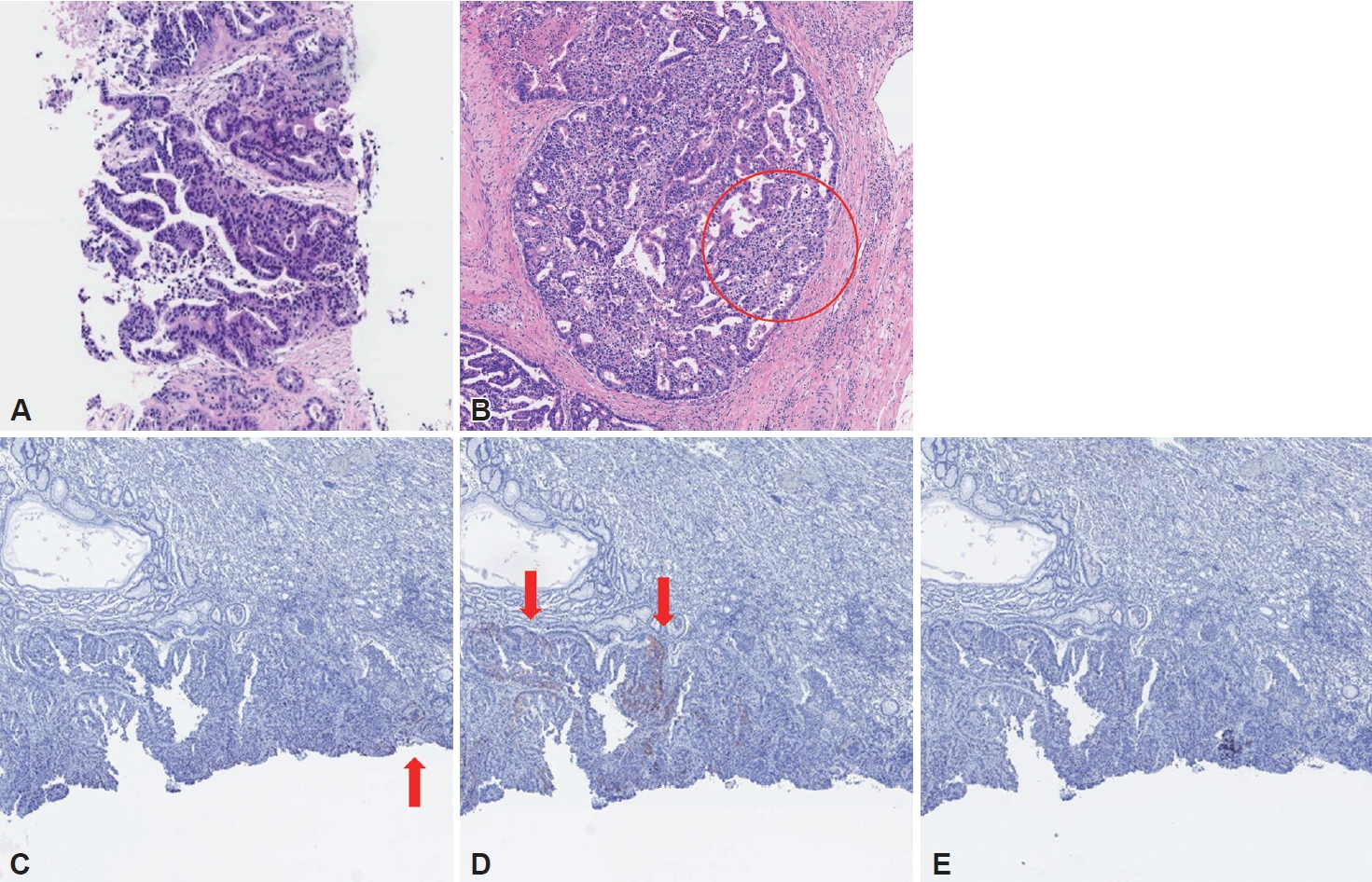
Histologic findings at the 6th month of follow-up. Percutaneous biopsy specimen of hepatic lesions. A: Biopsy specimen of the hepatic lesions showed adenocarcinoma, compatible for metastasis from the stomach (H&E, ×100). B: Some portions of the surgical specimen showed focal high grade changes in the nucleus with a clear cytoplasm (red circle), which suggested enteroblastic differentiation (H&E, ×100). The surgical specimen showed focal AFP (C) and SALL4 (D) expression, while the stain for glypican 3 (E) was negative (Immunohistochemistry, ×100). H&E, hematoxylin and eosin; AFP, alpha-fetoprotein; SALL4, sal-like protein 4.
The patient died of disease progression during palliative chemotherapy approximately 14 months after diagnosis with recurrence of GAED and hepatic metastases.
DISCUSSION
Histologically, GAED, hepatoid adenocarcinoma (HAC), and papillary adenocarcinoma are representative histologic subtypes of gastric adenocarcinoma with clear cells [7]. Among them, GAED and HAC shows variable expression of oncofetal proteins, while papillary adenocarcinoma is characterized and easily recognized by well-formed papillae [7]. According to a Japanese study, GAED is less frequent than CGA, accounting for approximately 2.2% of all GCs [1], and there are few largescale studies on GAED; therefore, its clinicopathological characteristics are not well understood [2]. In summary, GAED has a primitive intestine-like structure, histologically composed of cuboidal or columnar cells with a transparent cytoplasm [8,9]. GAED is also known to be associated with the production of AFP in the blood and tumor tissues and is associated with SALL4 and glypican 3, which are fetal gastrointestinal markers. Murakami et al. [3] have summarized GAED as an “adenocarcinoma composed of cells resembling the fetal gastrointestinal epithelium, with a pale cytoplasm, with immunopositivity for AFP, glypican 3, or SALL4.” However, not all GAEDs are positive for AFP, since there are reports of AFP-negative GAEDs [2,10]. Whether GAED develops from CGA or occurs sporadically has not been clearly established [11]. Recent studies have shown that SALL4 staining can diagnose GAED more sensitively than AFP [12]; the positive rate of AFP was 45% (13/29), whereas that of SALL4 was 72% (20/29) in a recent Japanese study comparing the two fetal gastrointestinal markers [3]. In addition, glypican 3 expression has been reported as a useful marker for the diagnosis of GAED [3].
Clinically, GAED is associated with rapid progression and poor prognosis [5,13], particularly with common lymphovascular invasion. Previous studies in Japan reported that 40% of early lesions and 84% of advanced lesions were accompanied by lymphatic invasion [4]. Hepatic metastasis is also known to be common in GAED as compared to CGA; there are reports of more than 30% of GAEDs that are accompanied by hepatic metastasis at diagnosis [3,5]. There are few studies on the prognosis of GAED in Korea; however, a study in 2019 reported that GAED showed a better prognosis than previously known, whereas HAC showed higher hepatic metastasis and recurrence rates [7]. They also reported that HAC showed characteristics distinct from GAED, including aggressive features and diffuse and strong expression of all oncofetal proteins [7]. These findings suggested the need for further large-scale studies on the clinical features and prognosis of GAED in Korea.
There are case reports of GAEDs treated with endoscopic submucosal dissection (ESD) in Japan [14,15]. In these cases, there was no specific feature in distinguishing GAED from CGA on endoscopy, narrow-band imaging, and endoscopic ultrasound, and no reported long-term follow-up results for more than a year. Therefore, special care should be taken when selecting ESD for the treatment of GAED, considering that previous studies have reported a high possibility of lymphovascular invasion or that this case observed rapid recurrence with extensive hepatic metastasis after radical resection. In addition, close follow-up for recurrence and metastasis is required if GAED is to be diagnosed after ESD.
This study had some limitations. First, serum AFP was not tested because AFP is not routinely tested in GC, and AFPproducing GC was not suspected at the time of initial diagnosis. Not all GAEDs produce AFP; however, in this case, additional staining for AFP in the pathological specimen of the primary carcinoma was positive. Therefore, it is thought that the possibility of GAED could have been predicted earlier if serum AFP had been tested at the time of the initial diagnosis of GC. In addition, only a part of the tumor tissue was positive for AFP and SALL4 staining, which was presumed to be due to technical problems, such as tissue fixation.
In conclusion, GAED has a high possibility of lymphovascular invasion and hepatic metastasis, showing rapid progression and poor prognosis; therefore, we reported a case in which care should be taken in the treatment and follow-up of GAED.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Yonghoon Choi, a contributing editor of the Korean Journal of Helicobacter and Upper Gastrointestinal Research, was not involved in the editorial evaluation or decision to publish this article.
Funding Statement
None
Ethics Statement
This study was reviewed and approved by the Institutional Review Board (IRB) of The Seoul National University Bundang Hospital (B-2402-880-701). Informed consent was waived by the IRB.
Acknowledgements
None
