|
 |
- Search
| Korean J Helicobacter Up Gastrointest Res > Volume 24(1); 2024 > Article |
|
Abstract
Ectopic pancreas refers to presence of pancreatic tissue in locations other than the pancreas (the usual anatomical site). This condition is often asymptomatic but can occasionally cause abdominal pain or other gastrointestinal symptoms. Ectopic pancreatic tissue is mainly observed in the stomach and duodenum and may be accompanied by complications, such as hemorrhage, pancreatitis, gastric outlet obstruction due to pseudocyst, and malignant transformation. We report a rare case of gastric ectopic pancreas accompanied by a pseudocyst that led to gastric outlet obstruction, which improved after endoscopic ultrasonography guided fine-needle aspiration in a young woman with habitual excessive alcohol consumption.
Ectopic pancreas is mainly located in the stomach and duodenum and is mostly discovered incidentally without symptoms. When symptoms are present, they are mostly nonspecific, such as epigastric pain. This condition undergoes various pathological changes that can also be observed in a normal pancreas, including bleeding [1], acute and chronic pancreatitis [2,3], cysts, and malignant transformation [4,5]. There have been reports of gastric ectopic pancreas causing gastric outlet obstruction (GOO) [6-9]. most of which were diagnosed and treated through surgery. Two cases of GOO caused by gastric ectopic pancreas were treated with endoscopic ultrasonography (EUS)-guided cystic drainage in China and Japan,6,8 one of which was observed without surgical resection and showed no signs of recurrence. To our knowledge, no previous report in Korea has described gastric ectopic pancreas with pseudocyst causing GOO, which was improved by EUS-guided cystic drainage. Here, we present a rare case of gastric ectopic pancreas associated with pancreatitis and pseudocyst, leading to GOO that presented with epigastric pain and vomiting in a young woman.
A 34-year-old woman presented with dyspepsia, epigastric pain, and vomiting that had begun one month prior. In the last month, she has complained of recurrent vomiting after each meal and has lost 2 kg of body weight. She had been a heavy drinker for three years, consuming 110 g alcohol daily, and a heavy smoker for 15 years, smoking 20 cigarettes daily. Her vital signs were unremarkable on initial outpatient examination. On physical examination, she complained of epigastric tenderness. Laboratory findings showed a significant elevation of aspartate aminotransferase (206 U/L; reference, 0 to 32 U/L), alanine aminotransferase (113 U/L; reference, 0 to 33 U/L), total bilirubin (1.33 mg/dL; reference, 0 to 1.2 mg/dL), and serum amylase (633 U/L; reference, 28 to 100 U/L). Serum lipase was within normal levels (53 U/L; reference, 13 to 60 U/L) and carcinoembryonic antigen (CEA) was slightly increased (5.2 ng/mL; reference, 0 to 4.3 ng/mL).
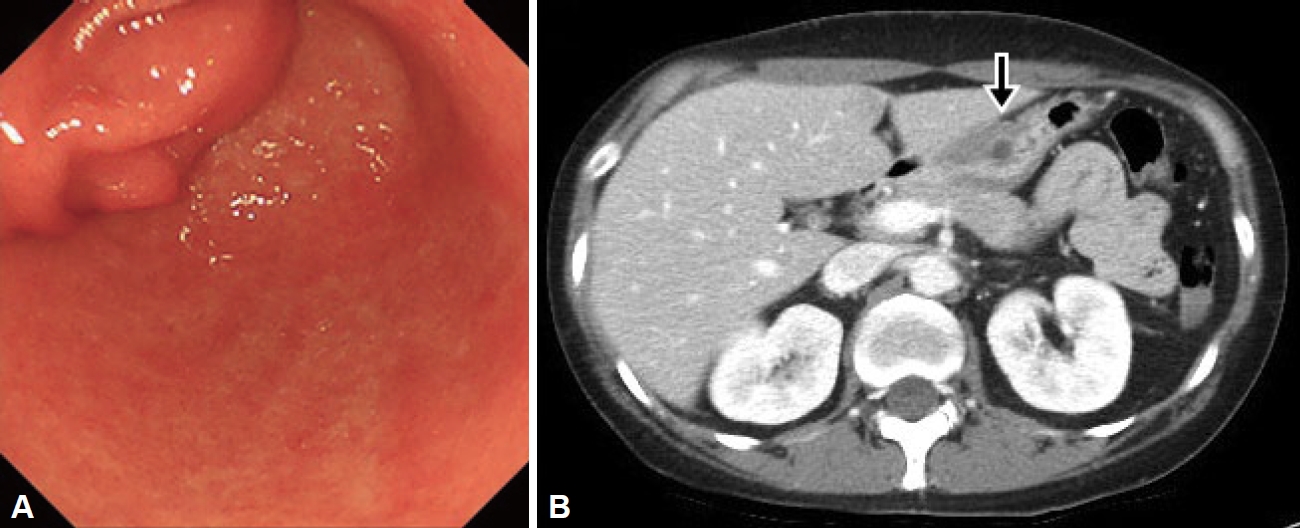
On upper endoscopy, a subepithelial lesion was found at the anterior wall in the distal antrum of the stomach with extrinsic compression on pylorus; its surface was covered by normal gastric mucosa, and a small opening was observed at its center. The pyloric channel was difficult to pass through with the Olympus Q290 endoscope (outer diameter 9.2 mm) (Fig. 1A). Abdominal computed tomography (CT) showed an approximately 4-cm, well-defined, low attenuation mass with relatively thin wall, with septation and focal septal calcification within the subepithelial portion of the gastric antrum (Fig. 1B). Based on the endoscopic and CT findings, EUS was performed and revealed a multi-cystic subepithelial lesion that seemed to originate from the third layer with internal hyperechoic materials and debris. Fine needle aspiration (FNA) and cystic drainage was performed (Fig. 2), and a total of 20 mL of serous fluid was aspirated. Cystic fluid cytologic examination showed numerous neutrophils with some lymphoid cells with no evidence of malignancy. The aspirated fluid amylase level was 965 U/L and the CEA level was 162 ng/mL. Based on these test results, GOO caused by gastric ectopic pancreas with pseudocyst formation was diagnosed.
The cystic lesion shrank after EUS-FNA and cystic drainage and the abdominal symptoms of GOO improved, after which the patient was discharged. Due to the possibility of malignant transformation and the patientŌĆÖs young age, periodic follow-up examinations were required, and surgical resection was discussed. However, the patient decided to only undergo followup observation. Follow-up endoscopy and CT performed six months later showed a decrease in the size of the lesion (Fig. 3). The patient is currently symptom-free, with no signs of recurrence of the lesion in endoscopic and CT examinations conducted every six months for two years.
In this report, we report a rare case of a large subepithelial cystic lesion arising from the ectopic pancreas successfully treated by EUS-FNA. This case emphasizes the need for heightened clinical awareness of gastric ectopic pancreas as a potential cause of gastrointestinal symptoms. EUS-FNA is considered as a valuable diagnostic and therapeutic tool in managing such cases, allowing for a precise diagnosis, as well as the treatment and improvement of symptoms.
Ectopic pancreas is a relatively common subepithelial lesion in the stomach, but it is not easy to differentiate from other subepithelial lesions. In particular, it is important to differentiate between pseudocysts arising from the gastric ectopic pancreas and intramural cystic lesions of the stomach. Gastric ectopic pancreas is characterized by central dimpling on the surface on endoscopic findings, and is mainly found in the prepyloric region of the stomach. The typical EUS findings of gastric ectopic pancreas include indistinct borders, heterogenous echogenicity, and an anechoic duct-like structure. When cystic lesions appear along with these characteristic lesions, they can be differentiated from intramural gastric pseudocysts such as gastric duplication cysts.
Although ectopic pancreas is often asymptomatic, it often gives rise to pseudocystic formation, pancreatitis, and malignant transformation. Pseudocyst formation in the ectopic pancreas develops when the secretory passage of the exocrine gland does not connect to the gastric mucosa, causing secreted enzymes to stagnate in the tissue, causing repetitive pancreatitis. The occurrence of acute pancreatitis in association with gastric ectopic pancreas adds a layer of complexity to its clinical presentation. Pancreatitis in this context may result from ductal obstruction, inflammation, or other factors related to the ectopic pancreatic tissue. Malignant transformation is a rare but known complication associated with ectopic pancreas [4,5]. Long-term surveillance and follow-up are essential in such cases, as malignant transformation may occur over time [5].
Several cases of acute pancreatitis with pseudocysts in the gastric ectopic pancreas were reported. Most cases were treated surgically, and chronic alcohol intake was not confirmed in the patients. Ryu et al. [10] reported pancreatitis and pseudocyst in the gastric ectopic pancreas of a patient with chronic alcohol consumption, and the condition improved with abstinence and conservative treatment. A case of gastric ectopic pancreas causing GOO in a chronic alcohol user with concomitant COVID-19 infection has been recently reported [11]. It is known that the toxic effects of alcohol can induce repetitive inflammation in the gastric ectopic pancreas. In our case, following EUS-FNA and abstinence, the patientŌĆÖs symptoms improved, and there was no recurrence thereafter. Therefore, the possibility of chronic alcohol consumption being the cause of pancreatitis and cystic formation cannot be ruled out.
Most pseudocysts in gastric ectopic pancreas are asymptomatic. However, if they are large or located near the pylorus, compression may cause a GOO. GOO due to ectopic pancreas with pseudocyst formation is rare, and most cases reported included a final diagnosis and treatment through surgical resection. However, since the possibility of malignant change is not high, surgical resection in cases where there is no recurrence and the possibility of malignancy is low has the disadvantage of reducing the quality of life. Two cases of GOO caused by gastric ectopic pancreas were treated with EUS-guided cystic drainage, and in the Japanese case [8], laparoscopic surgical resection was performed to confirm a histological diagnosis and prevent recurrence. In China, Jin et al. [6] were the first to report that a pseudocyst arising from gastric ectopic pancreas improved with only endoscopic treatment, and there were no signs of recurrence during four years of follow-up observation.
In our case, the patient exhibited symptoms of epigastric pain and vomiting, which were linked to GOO caused by the presence of a huge pseudocyst. The presented case of gastric ectopic pancreas with associated pancreatitis and pseudocyst highlights the complexities and challenges in the management of this rare condition. Ectopic pancreas, although generally asymptomatic, can lead to various complications that require a comprehensive understanding of its clinical implications. EUS-FNA played a crucial role not only in confirming the diagnosis but also in guiding therapeutic interventions. The drainage of the pseudocyst through EUS-FNA contributed significantly to the resolution of GOO and the improvement of the patientŌĆÖs symptoms.
In conclusion, we report a rare case of gastric ectopic pancreas with pseudocyst causing GOO, which improved with EUS-guided cystic drainage in a young woman with habitual heavy drinking. This case emphasizes the importance of a thorough evaluation and consideration of gastric ectopic pancreas in the differential diagnosis of GOO. EUS-FNA is considered as a valuable tool not only for accurate diagnosis but also for providing therapeutic interventions. Long-term followup and surveillance are crucial to monitor for potential complications, including malignant transformation, in patients with ectopic pancreas. Continued research and reporting of such cases will contribute to the evolving understanding of this rare condition and aid in refining clinical management strategies.
Notes
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
AuthorsŌĆÖ Contribution
Conceptualization: Jin Lee. Data curation: Jun Ho Kim. Investigation: Jun Ho Kim. Methodology: Ji Eun Na, Yong Eun Park. Supervision: Jin Lee, Jongha Park, Tae Oh Kim. WritingŌĆöoriginal draft: Jun Ho Kim. WritingŌĆöreview & editing: Jin Lee. Approval of final manuscript: all authors.
Fig.┬Ā1.
Endoscopic and CT images. A: Endoscopic image showing a large subepithelial lesion obstructing the pyloric ring. B: CT image showing a cystic tumor with a diameter of 4 cm, with minimal enhancement of the septal wall (arrow). CT, computed tomography.

REFERENCES
1. Joo YE, Kim HS, Choi SK, et al. Massive gastrointestinal bleeding caused by ectopic pancreas mimicking jejunal tumor. Digestion 2001;64:133ŌĆō136.



2. Elwir S, Glessing B, Amin K, Jensen E, Mallery S. Pancreatitis of ectopic pancreatic tissue: a rare cause of gastric outlet obstruction. Gastroenterol Rep (Oxf) 2017;5:237ŌĆō240.


3. Wawrzynski J, Leon L, Shah SA, Adrain A, Goldstein LJ, Feller E. Gastric heterotopic pancreas presenting as abdominal pain with acute and chronic pancreatitis in the resected specimen. Case Rep Gastrointest Med 2019;2019:2021712.




4. Lemaire J, Delaunoit T, Molle G. Adenocarcinoma arising in gastric heterotopic pancreas. Case report and review of the literature. Acta Chir Belg 2014;114:79ŌĆō81.


5. Jung HS, Lee J, Nam KH, et al. Gastric adenocarcinoma arising from heterotopic pancreas presenting as gastric outlet obstruction 10 years after the first diagnosis. Korean J Gastroenterol 2020;76:37ŌĆō41.


6. Jin HB, Lu L, Yang JF, et al. Interventional endoscopic ultrasound for a symptomatic pseudocyst secondary to gastric heterotopic pancreas. World J Gastroenterol 2017;23:6365ŌĆō6370.



7. Urade M, Fujimoto S. Ectopic pancreatitis in the antral stomach causing gastric outlet obstruction: a case of successful resection. Clin J Gastroenterol 2020;13:465ŌĆō471.



8. Yokoyama K, Sato H, Sato Y, Watanabe J, Nakamura A, Terai S. Rare gastric outlet obstruction owing to ectopic pancreas: a case report and literature review. Ann Transl Med 2020;8:252.



9. Dickerson A, Farrell A, Bushra A, et al. Gastric ectopic pancreas with pseudocyst formation causing gastric outlet obstruction. ACG Case Rep J 2023;10:e00988.



-
METRICS

-
- 0 Crossref
- 991 View
- 5 Download
- Related articles in Korean J Helicobacter Up Gastrointest Res
-
Gastric Outlet Obstruction by Impacted Phytobezoar at the Normal Duodenal Bulb2011 December;11(3)