Delayed Gastric Perforation by Nasogastric Tube: A Case Report
Article information
Abstract
Nasogastric (NG) tube insertion is a common and routine procedure that is performed for various purposes. Gastric perforations caused by NG tubes are rare but potentially life-threatening. We report a rare case involving a delayed gastric perforation caused by an NG tube in an adult with end-stage heart failure.
INTRODUCTION
Nasogastric tube (NG tube) insertion is a common and routine procedure with various purposes including gastric decompression, irrigation, and/or nutritional support, and it is considered a safe procedure [1]. The gastric perforation by the NG tube is a rare and potentially life-threatening condition. A few cases of the perforations of the alimentary tract by the NG tube insertion have been reported and NG tube-related gastric perforations have been reported frequently in neonates and infants [2-4]. Delayed gastric perforation by NG tube is even rarer [5,6]. We report a rare case of a delayed gastric perforation by the NG tube in an adult patient with end-stage heart failure.
CASE REPORT
A 66-year-old male patient presented with a chief complaint of aggravating dyspnea on exertion (New York Heart Association classification III), with a whole-body edema of a 5 kg increase in body weight. He had a complex medical history of pulmonary tuberculosis, hip arthroplasty, stroke, lung cancer, pacemaker insertion for complete atrioventricular block and atrial fibrillation, implantation of cardiac resynchronization therapy-defibrillator, and tricuspid valve repair. During his hospital stay in cardiology general ward, he underwent medical management for acute heart failure, decompensated alcoholic cirrhosis, vertebral body compression fracture, cellulitis, Clostridium difficile infection, sepsis, and acute kidney injury.
As his condition deteriorated due to the aggravation of heart failure, he was transferred to the intensive care unit (ICU) on hospital day 101 (ICU Day 1). Continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO) were initiated on ICU Day 2 and 5, respectively. He was initially fed orally but started enteral feeding via the NG tube with the initiation of ECMO (ICU Day 5). On ICU Day 8, the NG tube was removed by the patient due to delirium, and an 18 French gauze NG tube was successfully reinserted on ICU Day 11 as his delirium improved (Fig. 1). Since the patient’s delirium was improved, the NG tube insertion was done manually without specific event. Following NG tube reinsertion, he developed hospital-acquired pneumonia showing a total whiteout of the left lung, requiring three sessions of bronchoscopic toileting at ICU Day 15, 17, 18.

X-ray images of the NG tube. A: Immediately after manual NG tube insertion (ICU Day 11). B: The day after NG tube insertion (ICU Day 12). C: The day prior to the onset of peritoneal signs (ICU Day 19). D: The day when peritoneal sign develop. NG tube, nasogastric tube; ICU, intensive care unit.
Despite these supportive cares, he developed acute abdominal pain with peritoneal signs on ICU Day 20, leading to the cessation of tube feeding. The computed tomography imaging revealed a definite gastric perforation by the NG tube (Fig. 2). In a multidisciplinary discussion, the risk of surgical intervention was deemed too high due to his critical condition and ongoing ECMO therapy, so an endoscopic intervention was decided instead of surgery. Endoscopic examination using carbon dioxide gas confirmed the gastric perforation of the high body/greater curvature by the NG, and the size of gastric perforation was identical to the diameter of the tube (Fig. 3A). There was no evidence of gastric ulcer, gastric erosion, or ischemic insult. Some amounts of food materials were drained from the peritoneal cavity to the stomach lumen via the perforation site immediately after removing the NG tube (Fig. 3B). The gastric perforation was managed with clipping using a total of five clips, and a new NG tube was placed in the antrum applying negative pressure. Despite the active and aggressive management, the patient deteriorated rapidly with abrupt elevation of inflammation markers and oxygen demand. Decompensated heart failure, hospital pneumonia and peritonitis due to tube feeding material led to cardiogenic shock, respiratory failure, and septic shock simultaneously. Even with the support of ECMO and CRRT, the patient expired on ICU Day 24, three days after the endoscopic management, marking a total of 124 days in the hospital.
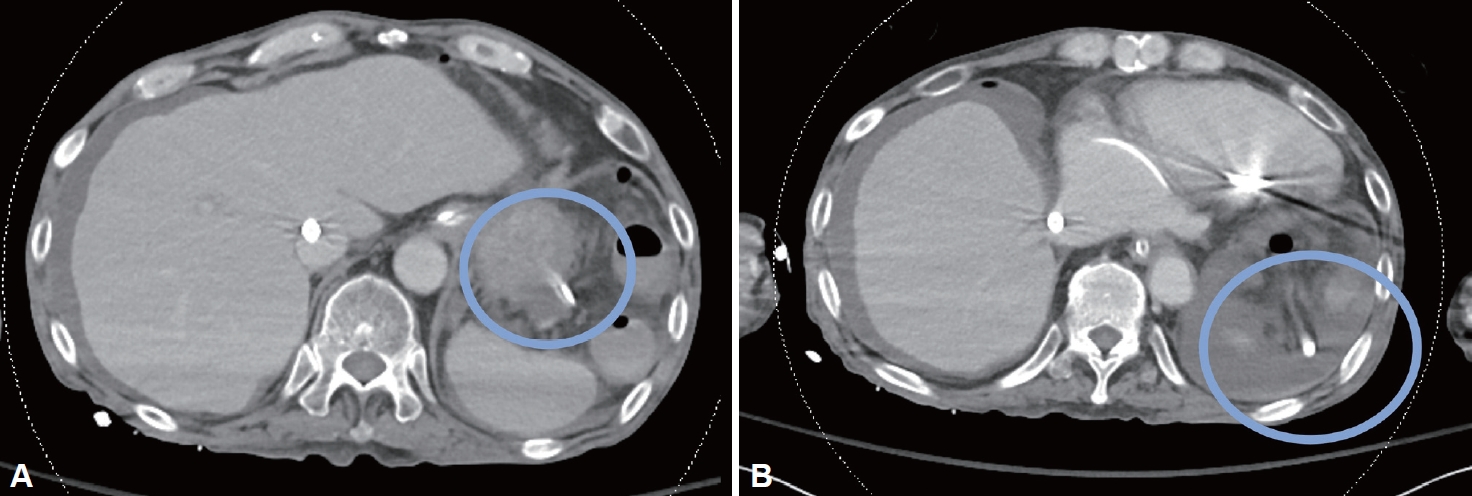
The gastric perforation by the NG tube was revealed in the computed tomography. A: The NG tube is penetrating the stomach wall. B: The NG tube tip is positioned outside the stomach near the spleen. NG tube, nasogastric tube.
DISCUSSION
Gastric perforation in adults typically results from peptic ulcers, gastric cancer, or trauma. Conversely, esophageal perforation by NG tube insertion is more common than gastric perforation by NG tube insertion in adult patients, and it is associated with factors such as multiple insertion attempts, preexisting anatomic abnormalities of the esophagus, altered mental status, cardiomegaly, concurrent tracheal intubation, and the presence of cervical osteophytes [7,8]. Cases of NG tube-induced gastric perforation have been reported predominantly in neonates and infants, and such instances are notably rare in adults.
The cases involving existing NG tubes and delayed gastric perforation are even rarer than cases of new insertions [4]. There are only two previous case reports and a case series of delayed gastric perforation by NG tube reported in English literature [5,6,9]. A case series reported in 1980 had only two cases of delayed gastric perforation by NG tube with limited clinical information [9]. We were not able to identify risk factors by any statistical analysis, but a commonality was impressive: all three patients of the three case reports including our current case report underwent ICU care for cardiogenic shock with radiographic evidence of the NG tube’s tip located at the gastric fundus, and their NG tubes were maintained more than 5 days until gastric perforation occurs (Table 1). We could assume that the presence of cardiogenic shock, the positioning of the NG tube’s tip in the gastric fundus and retaining NG tube for more than five days may be potential risk factors.
In the case series report in 1980, it was suggested that the degradation of the NG tube retaining for more than five days by gastric acid could lead to color changes and increased tube rigidity, subsequently causing perforation [9]. Susmallian et al. [10], reported data of the stomach wall thickness of 100 patients, with accurate measurements taken separately for the fundus, body, and antrum using a digital caliper immediately after sleeve gastrectomy. The stomach wall thickness were 5.1±0.6 mm for the antrum, 4.1±0.6 mm for the body, and 2.6±0.5 mm for the fundus, and the anterior part was thicker than the posterior part for all areas. These previous reports support the hypothesis that retention of NG tube for more than five days and positioning of NG tube in gastric fundus are risk factors of delayed gastric perforation by NG tube. Further analysis of additional cases is necessary to confirm this hypothesis. Other possible reasons for spontaneous delayed gastric perforation include peptic ulcer disease, gastric malignancy, nonsteroidal anti-inflammatory medication or gastric ischemia [11].
In this case report, the patient was critically ill patient with end-stage heart failure and multiple comorbidities, and the endoscopic exam revealed neither ulcerative lesion nor evidence of ischemia around the perforation site. And, in a retrospective view, the daily radiographic examinations of the patient showed an unusual location of gastric fundus and an unusual caudal direction of the NG tube without changes for several days. We could assume that the tip of NG tube had been pressing the greater curvature of stomach high body wall and observed as if its location is the gastric fundus, and the only explainable mechanism for the gastric perforation in this case was the prolonged pressure by the rigidified NG tube against the thin greater curvature and posterior wall of high body, since other causes like gastric ulcer or erosion were excluded by the endoscopic examination. A meticulous evaluation of the routine radiographic findings, timely adjustment of the location and direction of the NG tube, and relief of the pressure on the stomach wall might have prevented the perforation.
In conclusion, delayed gastric perforation by the NG tube is a rare but possible lethal adverse event of NG tube insertion. We report a rare case of a delayed gastric perforation by the NG tube in an adult patient with end-stage heart failure. Presence of cardiogenic shock, positioning of NG tube’s tip in thin gastric fundus and retention of NG tube for more than five days are hypothesized as potential risk factors of this adverse event. To prevent delayed gastric perforation for patients with multiple comorbidities, it is recommended to insert NG tube gently, ensuring its tip aligns with the greater curvature wall, rather than perpendicular in the gastric fundus, and to maintain this alignment.
Notes
Availability of Data and Material
All data in this article are available from the corresponding author on reasonable request.
Conflicts of Interest
Soo-Jeong Cho, the Editor-in-Chief of the Korean Journal of Helicobacter and Upper Gastrointestinal Research, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
None
Authors’ Contribution
Writing—original draft: Junyeol Kim. Writing—review & editing: SooJeong Cho. Approval of final manuscript: all authors.
Ethics Statement
This study was reviewed approved by the Institutional Review Board of the Seoul National University Hospital (IRB number 2311-146-1487).
Acknowledgements
None


