INTRODUCTION
Helicobacter pylori is a gram-negative, spiral-shaped bacterium that colonizes the stomach, predominantly in the antral region. Infection with this organism is asymptomatic in most individuals. However, such infections have been shown to cause numerous gastric pathologies, including gastritis, peptic ulcer disease, adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma [
1]. Therefore, the accurate diagnosis and treatment of these infections is essential.
Three studies have investigated the prevalence of
H. pylori infections in the South African population. The estimated prevalence is 66%–76% in the KwaZulu-Natal Province [
2,
3] and 86.8% in the Eastern Cape Province [
4]. Overall, these studies showed a higher prevalence than that reported globally (48.5%) [
5].
Diagnostic procedures for confirming
H. pylori infection can be divided into two broad categories: invasive and noninvasive. The three noninvasive tests for
H. pylori that are primarily used, currently, are the urease breath test, stool antigen test, and serological test [
6]. The most commonly used noninvasive test is the urease breath test. This test utilizes the innate ability of the bacteria to produce urease, which helps neutralize the gastric acid in the surrounding environment. The test is used both for diagnosing an
H. pylori infection and for determining if the bacteria have been eradicated following therapy [
6].
The rapid urease test is the fastest invasive method for diagnosing an
H. pylori infection. This test, which is similar to the urease breath test, relies on production of urease by the infecting bacteria. The test is performed under direct gastroscopy, which also allows for visualizing any macroscopic lesions present in the stomach mucosa. This test is relatively inexpensive and highly specific, with studies showing specificities of 95%–100%. However, this test has low sensitivity (85%–95%) and is not reliable for excluding an
H. pylori infection [
7].Although
H. pylori can be cultured to diagnose an infection, the long turnaround time and intensive labor involved mean that the test is usually reserved for determining drug sensitivity [
7]. Polymerase chain reaction techniques have been introduced to help determine both the presence and clarithromycin resistance pattern of
H. pylori [
8]. This method has been successfully used for stool samples and has a sensitivity of 91% [
9].
Histologic evaluation, another invasive method, uses various staining techniques and light microscopy to visualize the presence of
H. pylori. The routinely performed hematoxylin and eosin (H&E) histochemical staining method has been shown to detect
H. pylori with a sensitivity of 83%–91% [
10-
12]. Therefore, many institutions consider H&E staining to be sufficient for detecting
H. pylori in the majority of cases and further recommend that other stains should not be routinely performed [
12-
14]. However, scrutinizing a H&E stain for
H. pylori organisms is a time-consuming exercise that is prone to interobserver variability. Due to factors such as turnaround time and pathologist preference, some institutions have opted to routinely utilize additional stains upfront [
15]. Additional stains that can be used include both histochemical and immunohistochemical (IHC) stains.
The histochemical stains used for
H. pylori detection include the modified Giemsa (MG), Diff-Quik, cresyl violet (CV), and Warthin–Starry stains. MG staining is the most commonly used because it is relatively inexpensive and technically easy to perform [
16]. A study that compared the sensitivity of CV to that of MG showed that CV staining had superior sensitivity (76%) compared with MG staining (68%) [
17]. These stains have also been shown to have variable sensitivities based on the amount of gastric activity present. For example, the sensitivity of MG staining is as low as 36% in the absence of gastric activity. However, in the presence of active chronic gastritis with structural alteration, the sensitivity is 92% [
18].
The Japanese guidelines for managing
H. pylori infection recommend the routine use of H&E and MG stains to detect the bacteria. IHC is only recommended in specific scenarios, such as a low bacterial load and the presence of coccoid bacteria [
19]. Our laboratory performed routine CV and IHC stains for all gastric biopsies during the year preceding the study owing to turnaround time and workflow management pressures.
Studies have shown that the
H. pylori load can alter detection sensitivity; however, the sensitivity of CV staining at different bacterial loads has not been specifically investigated [
20]. Moreover, no studies have compared the effectiveness of CV staining for detecting
H. pylori in patients with and without gastric activity.
IHC staining has been shown to be the superior staining method for diagnosing
H. pylori infections, with sensitivities of 97%–100% [
10]. However, the main drawback of this method is its high cost, especially when routinely performed [
15]. To date, studies have not compared the time taken to identify
H. pylori using IHC and histochemical stains.
This study aimed to determine the average time required to identify H. pylori infection, compare sensitivities at different bacterial loads, and determine the influence of gastric activity on results reliability for both CV and IHC staining procedures.
METHODS
Cases were retrospectively identified by searching the laboratory information system of a private histopathology practice in Cape Town, South Africa. One thousand consecutive cases, assessed beginning January 1, 2021, were selected for analysis. CV staining was performed on tissue sections (3–4 μm thickness) with a commercially available CV solution (Merck, Darmstadt, Germany), as previously described [
21]. In all cases, IHC staining was performed using
H. pylori antibody diluted 1:200 (mouse monoclonal, clone ULC3R; Novocastra, Newcastle upon Tyne, United Kingdom).
This study was approved by the University of Cape Town Human Research Ethics Committee (HREC 209/2022) and Stellenbosch University (N22/07/075_RECIP_UCT_209/2022).
Electronic histopathology reports were reviewed for each case, and the following information was recorded: diagnosis; activity (mild, moderate, or severe); chronic changes (present or absent);
H. pylori load, if present (mild, moderate, or severe); and intestinal-type metaplasia (present or absent). The updated Sydney System was used to categorize chronic gastritis [
22] as non-atrophic, atrophic, or special; the special forms include chemical, radiation-associated, and lymphocytic gastritis.
Patients that were reported as having normal, chemical gastritis or who were diagnosed with polyps only were excluded. Cases in which slides were not available or were uninterpretable were also excluded. The retrieved CV-stained and IHC slides were examined independently by three consultant histopathologists. The presence or absence of H. pylori was recorded, as was the time taken to reach each conclusion. The slides from each case were examined separately (i.e., not sequentially) to avoid bias. The presence or absence of H. pylori recorded in the original histopathology report was used as the ground truth.
Receiver operating characteristic (ROC) curve analysis was used to calculate the p-values for the sensitivity and specificity of the diagnostic tests. Cohen’s kappa value was determined to assess the interobserver agreement between the pathologists who examined the samples.
RESULTS
Of the 1000 sequentially identified cases, 513 were excluded due to diagnoses of non-
H. pylori-related conditions. Among the excluded cases, 241 were considered normal, 230 involved diagnoses of chemical gastropathy, and 42 were diagnosed as gastric polyps (
Table 1). Of the included 487 cases, 275 represented a diagnosis of inactive chronic gastritis (182 nonspecific and 93 follicular), 183 active gastritis, 14 atrophic gastritis, 8 ulcer tissue only, and 7 gastric malignancy.
H. pylori was diagnosed in 237 of the 1000 cases (prevalence 23.7%).
A total of 443 CV-stained and 451 IHC slides were assessed in this study. Of the 443 CV slides, 258 were evaluated by at least two pathologists. Of the 451 IHC slides, 283 (118 positive for H. pylori) were examined by at least two pathologists. There were 14 cases in which the H. pylori were missed by both pathologists reading the CV-stained sections but were detected by both pathologists reading the IHC sections. Of these 14 cases, 12 had low H. pylori loads, two showed coccoid forms, and 11 were from patients diagnosed with inactive chronic gastritis. Conversely, there were no cases of H. pylori infection that were missed by both pathologists reading IHC-stained sections but diagnosed by both pathologists reading the CVstained sections.
The mean time to evaluate a CV slide was 48.9 (range 45.6– 61.7) seconds compared with 15.8 (range 10.5–34.4) seconds for an IHC slide (
Table 2). In this study, the overall sensitivity of CV staining was 64.5% compared with 85.2% for IHC staining (
Table 3). The average sensitivity for CV staining was 33.7% at a low
H. pylori load; where none of the pathologists demonstrated a sensitivity greater than 35%. For the IHC slides, the average sensitivity was 67.0% at low
H. pylori loads.
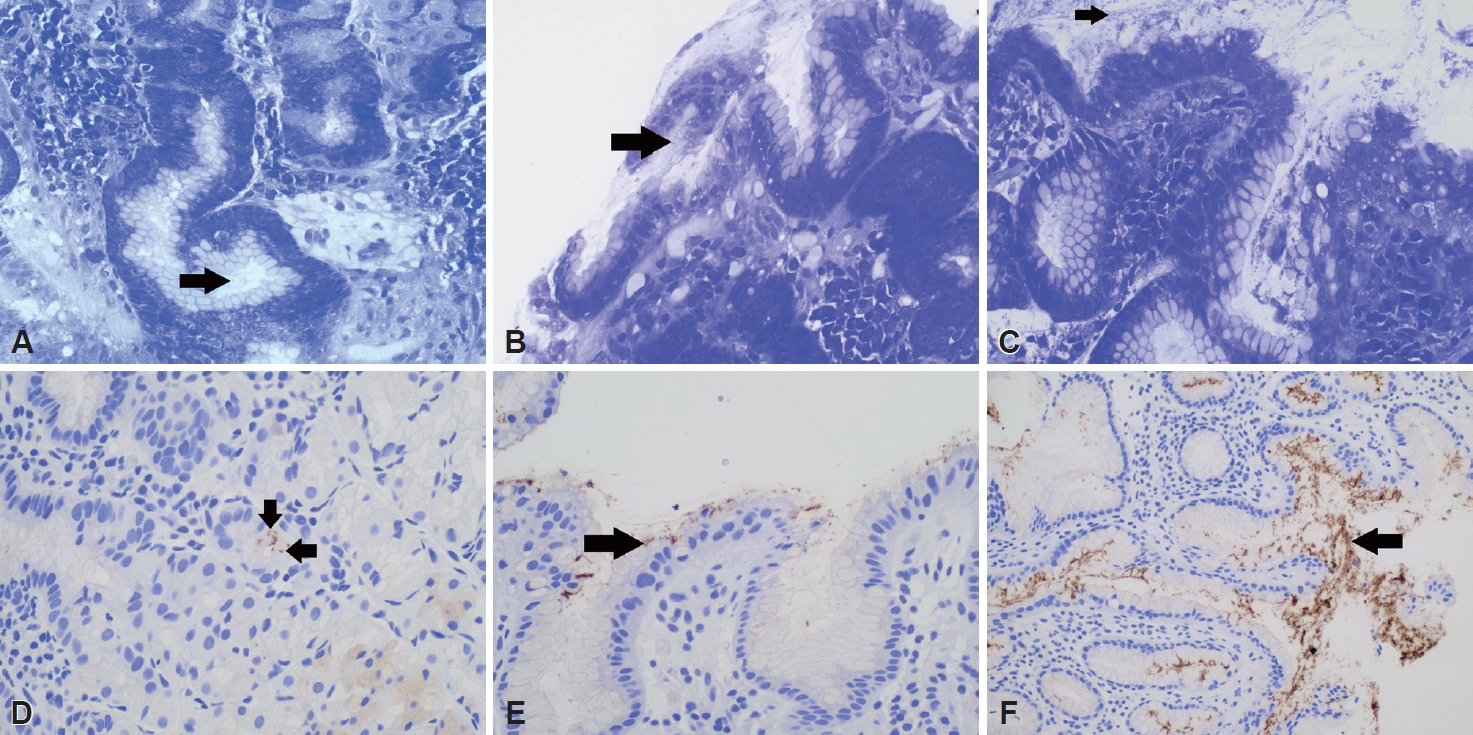
Fig. 1 shows the corresponding CV and IHC staining results for cases with low, moderate, and high
H. pylori loads. The ROC analyses indicated that, at high
H. pylori loads, all pathologists demonstrated good diagnostic ability using both CV-stained and IHC sections (all, p<0.05). At low
H. pylori loads none of the pathologists demonstrated a better than chance diagnostic ability using either staining method (all,
p>0.05).
Table 4 shows the high sensitivity and specificity of IHC staining for detecting
H. pylori in patients with both active and chronic inactive gastritis. CV staining demonstrated particularly poor sensitivity for detection in patients with chronic inactive gastritis, with sensitivities of 52.0% (chronic follicular gastritis) and 57.8% (chronic nonspecific gastritis). The sensitivities and specificities of IHC and CV staining were both 100% in cases involving patients with atrophic gastritis.
Cohen’s kappa statistic was used to compare the interobserver agreement among the three pathologists. For CV staining, the kappa value was 0.605 (substantial agreement); for IHC staining, the value was 0.568 (moderate agreement).
DISCUSSION
The prevalence of
H. pylori in our study was lower than that described in three previously published studies from South Africa and is lower than the reported global prevalence [
5]. This finding was unexpected, as we hypothesized that patients who are symptomatic and undergo gastroscopy and biopsy would have a higher prevalence of
H. pylori infection. However, the observed results may be due to the National Guidelines of South Africa, which recommend that patients undergo eradication therapy before gastroscopy if
H. pylori infection is clinically suspected [
23]. Hence, the numbers of samples that represent successful eradications of previous
H. pylori infection versus samples from patients without prior
H. pylori infection cannot be determined. Additionally, the study population exclusively represents private-sector patients from the Western Cape who have ready access to endoscopy and who may also undergo gastroscopy for other indications, such as symptoms of gastroesophageal reflux disease.
The evaluation of CV-stained samples took an average of three times longer than did that of IHC slides. As expected, the slides that were positive for H. pylori were quicker to evaluate using either CV or IHC staining than those that were negative. Reaching a conclusion that a sample was H. pylori-positive was more than four times (4.3) faster for IHC samples than for those stained with CV; concluding that a sample was H. pylori-negative was slightly less than three times (2.9) quicker using IHC. This can be explained by the fact that the color contrast of IHC stained tissue sections, at low-power magnification (brown against white), is more readily identifiable than that of CV-stained tissue sections (blue on blue) where a pathologist also needs to identify the shape of the organism at high-power magnification. When an organism is not readily identifiable with CV, a careful search and evaluation of debris, mucin, and other nonspecific bacteria are required before confidently concluding the absence of H. pylori.
The practical implications of this finding imply that histopathology laboratories that use routine IHC staining spend less time interpreting each case than those that use routine CV staining. This study also demonstrated that a pathologist could save approximately 30 seconds per case (slide) when using IHC-stained sections compared with CV-stained sections. This means that for every 1000 slides the pathologist examines, roughly one working day (approximately 8 hours) would be saved if IHC staining was routinely used rather than CV staining.
To the best of our knowledge, this is the first study to determine the time taken to diagnose H. pylori infection using different staining methods. There are no published references for the average amount of pathologist time required to determine a patient’s H. pylori infection status; therefore, our results cannot be compared with any existing standards. These results may be useful as novel benchmarks for the time required to diagnose H. pylori infections using IHC and histochemical stains.
IHC staining has been shown to be more reliable than other non-IHC methods for detecting
H. pylori, particularly in the setting of scant and/or coccoid organisms. Histochemical stains highlight all the bacterial organisms present; therefore, careful assessment of the characteristic
H. pylori shape and size is required. The reported sensitivities of histochemical methods range between 60% and 90%, which is similar to our determined sensitivity of 64.5% [
10,
24-
26].
Our findings show that CV staining is a poor ancillary diagnostic test for detecting
H. pylori, particularly when there is a low bacterial load present. IHC staining showed a sensitivity almost double that of CV staining (67.0% vs. 33.7%) at a low organism load. In addition, of the 14
H. pylori-positive cases missed by the two pathologists using CV staining but diagnosed by both pathologists using IHC staining, 12 had low
H. pylori loads. This is a further indication that CV staining is less effective than IHC staining at detecting
H. pylori in patients with low organism loads. This is not surprising, as
H. pylori are known to sequester deep within the gastric pits when they are present at low densities, making them more difficult to detect using histochemical stains. Another explanation for this discrepancy is that some patients may have been previously treat-ed with antibiotics, which can also cause
H. pylori to assume an inactive, coccoid morphology that is detectable only by IHC staining [
27]. In the present study, the sensitivities of both staining methods improved, for all pathologists, as the bacterial load increased. Although no previous study has directly examined CV staining performance at different
H. pylori loads, we expect it to have a similar performance as other histochemical stains. In addition, the ROC analysis showed that, at low
H. pylori loads, CV and IHC staining methods demonstrated similar performances. This suggests that although IHC staining is superior to CV staining at low
H. pylori loads, neither can be considered a reliable test for excluding the possibility of an infection in this scenario.
Not only did IHC staining have superior sensitivity, but all pathologists demonstrated higher specificity using this method than when using CV staining (97.7% vs. 90.6%). Furthermore, in the subset of samples that were examined by two pathologists, 11.9% (14/118) of cases were missed by both pathologists using the CV stain but were identified by both pathologists using the IHC stain.
The diagnostic sensitivity of staining to detect
H. pylori was higher in the presence of active gastritis. Moreover, inactive chronic follicular gastritis resulted in a lower sensitivity for both stains. This finding is consistent with those of other studies that have shown
H. pylori detection sensitivity improves when active gastritis is present [
18]. This is a potentially useful finding as it cautions pathologists to consider examining additional tissue sections or using additional stains when a diagnosis of inactive follicular gastritis is made without evidence of
H. pylori.
Interestingly, there was similar interobserver agreement between pathologists when using the CV (0.61) and IHC (0.57) methods. The surprisingly low kappa value for the IHC method may be due to different levels of experience and familiarity of the pathologists with this stain.
The overall sensitivity of IHC staining for detecting the presence of
H. pylori, in our study, was lower than that reported in other international studies (85.2% vs. 97%–100%) [
10]. This is most likely due to the study design and exposes some of its weaknesses. In the present study, the ground truth was accepted to be the presence or absence of
H. pylori recorded in the original histopathological report. Pathologists originally reporting the cases had the advantages of having the clinical history from the referring endoscopist, multiple extra tissue sections to examine, and access to all slides from all specimens sent for each patient. The pathologists in this study were blinded to the clinical history and only had access to the histochemical and IHC slides for each case. Although these factors may have lowered the sensitivity, the variables were the same across the all sample interpretations; hence, the comparison between the two staining methodologies is reliable.
CV staining is a suboptimal ancillary diagnostic test for identifying the presence of H. pylori, with a low overall sensitivity (64.5%) and even worse performance in samples from patients with low organism loads and inactive gastritis. IHC staining was shown to have higher sensitivity (85.2%) than CV staining in samples from patients with these conditions. The use of IHC staining as a first-line ancillary test allowed detection of cases that were missed using CV staining and the interpretation was, on average, three times faster than for CV staining. This is relevant in the setting of high caseloads and pressurized turnaround times. Therefore, if cost permits, IHC staining is recommended over CV staining as a diagnostic test for H. pylori infections. Moreover, in a resource-limited setting, IHC staining, should be considered over CV staining for samples from patients with inactive chronic gastritis.